Herpes simplex virus 1 precludes replenishment of the short-lived receptor of tumor necrosis factor alpha by virion host shutoff-dependent degradation of its mRNA
- PMID: 16840355
- PMCID: PMC1563695
- DOI: 10.1128/JVI.00587-06
Herpes simplex virus 1 precludes replenishment of the short-lived receptor of tumor necrosis factor alpha by virion host shutoff-dependent degradation of its mRNA
Abstract
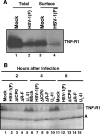
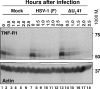
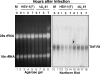
Cell function is tightly regulated by surface receptors. Earlier reports showed that herpes simplex virus 1 regulates by diverse mechanisms the presentation of antigenic peptides, downregulates the signaling pathways associated with receptor tyrosine kinases, and posttranslationally modifies members of the Src family of protein kinases. Here we report that the receptor for tumor necrosis factor alpha (TNF-R1) rapidly disappears from both the cell surface and total cell lysates in cells infected with wild-type virus or a variety of mutants but not in cells infected with the mutant DeltaU(L)41, which lacks the U(L)41 gene, the virion host shutoff gene. The half-life of TNF-R1 appears to be less than 30 min in both mock-infected and infected cells. The disappearance of TNF-R1 correlates with the disappearance of cytoplasmic TNF-R1 mRNA in wild-type-virus-infected cells. The results suggest that by degrading the TNFR1 mRNA, the virus precludes the replenishment of naturally decaying TNF-R1.
Figures

Similar articles
-
Infected cell protein 22 of herpes simplex virus 1 regulates the expression of virion host shutoff gene U(L)41.Virology. 1997 Aug 4;234(2):226-34. doi: 10.1006/viro.1997.8659. Virology. 1997. PMID: 9268153
-
Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs.J Virol. 2004 Aug;78(16):8582-92. doi: 10.1128/JVI.78.16.8582-8592.2004. J Virol. 2004. PMID: 15280467 Free PMC article.
-
The herpes simplex virus type 1 vhs-UL41 gene secures viral replication by temporarily evading apoptotic cellular response to infection: Vhs-UL41 activity might require interactions with elements of cellular mRNA degradation machinery.J Virol. 2006 Jan;80(1):505-13. doi: 10.1128/JVI.80.1.505-513.2006. J Virol. 2006. PMID: 16352574 Free PMC article.
-
Early shutoff of host protein synthesis in cells infected with herpes simplex viruses.Acta Virol. 2001;45(5-6):269-77. Acta Virol. 2001. PMID: 12083325 Review.
-
Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase?J Virol. 2004 Feb;78(3):1063-8. doi: 10.1128/jvi.78.3.1063-1068.2004. J Virol. 2004. PMID: 14722261 Free PMC article. Review. No abstract available.
Cited by
-
Small interfering RNAs that deplete the cellular translation factor eIF4H impede mRNA degradation by the virion host shutoff protein of herpes simplex virus.J Virol. 2008 Jul;82(13):6600-9. doi: 10.1128/JVI.00137-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448541 Free PMC article.
-
Expression of gamma interferon-dependent genes is blocked independently by virion host shutoff RNase and by US3 protein kinase.J Virol. 2008 May;82(10):4688-96. doi: 10.1128/JVI.02763-07. Epub 2008 Mar 5. J Virol. 2008. PMID: 18321964 Free PMC article.
-
Relative Contributions of Herpes Simplex Virus 1 ICP0 and vhs to Loss of Cellular IFI16 Vary in Different Human Cell Types.J Virol. 2016 Aug 26;90(18):8351-9. doi: 10.1128/JVI.00939-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27412599 Free PMC article.
-
The latency-associated UL138 gene product of human cytomegalovirus sensitizes cells to tumor necrosis factor alpha (TNF-alpha) signaling by upregulating TNF-alpha receptor 1 cell surface expression.J Virol. 2011 Nov;85(21):11409-21. doi: 10.1128/JVI.05028-11. Epub 2011 Aug 31. J Virol. 2011. PMID: 21880774 Free PMC article.
-
Extracellular Vesicles Released by Herpes Simplex Virus 1-Infected Cells Block Virus Replication in Recipient Cells in a STING-Dependent Manner.J Virol. 2018 Aug 29;92(18):e01102-18. doi: 10.1128/JVI.01102-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976662 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

