Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release
- PMID: 16840518
- PMCID: PMC1995637
- DOI: 10.1113/jphysiol.2006.111336
Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release
Abstract
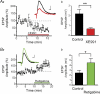
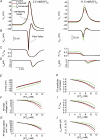
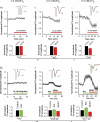
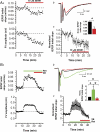
M-current (I(M)) plays a key role in regulating neuronal excitability. Mutations in Kv7/KCNQ subunits, the molecular correlates of I(M), are associated with a familial human epilepsy syndrome. Kv7/KCNQ subunits are widely expressed, and I(M) has been recorded in somata of several types of neurons, but the subcellular distribution of M-channels remains elusive. By combining field-potential, whole-cell and intracellular recordings from area CA1 in rat hippocampal slices, and computational modelling, we provide evidence for functional M-channels in unmyelinated axons in the brain. Our data indicate that presynaptic M-channels can regulate axonal excitability and synaptic transmission, provided the axons are depolarized into the I(M) activation range (beyond approximately -65 mV). Here, such depolarization was achieved by increasing the extracellular K(+) concentration ([K(+)](o)). Extracellular recordings in the presence of moderately elevated [K(+)](o) (7-11 mm), showed that the specific M-channel blocker XE991 reduced the amplitude of the presynaptic fibre volley and the field EPSP in a [K(+)](o)-dependent manner, both in stratum radiatum and in stratum lacknosum moleculare. The M-channel opener, retigabine, had opposite effects. The higher the [K(+)](o), the greater the effects of XE991 and retigabine. Similar pharmacological modulation of EPSPs recorded intracellularly from CA1 pyramidal neurons, while blocking postsynaptic K(+) channels with intracellular Cs(+), confirmed that active M-channels are located presynaptically. Computational analysis with an axon model showed that presynaptic I(M) can control Na(+) channel inactivation and thereby affect the presynaptic action potential amplitude and Ca(2+) influx, provided the axonal membrane potential is sufficiently depolarized. Finally, we compared the effects of blocking I(M) on the spike after-depolarization and bursting in CA3 pyramidal neuron somata versus their axons. In standard [K(+)](o) (2.5 mm), XE991 increased the ADP and promoted burst firing at the soma, but not in the axons. However, I(M) contributed to the refractory period in the axons when spikes were broadened by a low dose 4-aminopyridine (200 microm). Our results indicate that functional Kv7/KCNQ/M-channels are present in unmyelinated axons in the brain, and that these channels may have contrasting effects on excitability depending on their subcellular localization.
Figures


Similar articles
-
M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region.J Neurosci. 2007 Feb 21;27(8):1853-67. doi: 10.1523/JNEUROSCI.4463-06.2007. J Neurosci. 2007. PMID: 17314282 Free PMC article.
-
Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells.J Physiol. 2005 Aug 1;566(Pt 3):689-715. doi: 10.1113/jphysiol.2005.086835. Epub 2005 May 12. J Physiol. 2005. PMID: 15890705 Free PMC article.
-
Pharmacological modulation of the voltage-gated neuronal Kv7/KCNQ/M-channel alters the intrinsic excitability and synaptic responses of pyramidal neurons in rat prefrontal cortex slices.Acta Pharmacol Sin. 2017 Sep;38(9):1248-1256. doi: 10.1038/aps.2017.72. Epub 2017 Jun 12. Acta Pharmacol Sin. 2017. PMID: 28603289 Free PMC article.
-
Neural KCNQ (Kv7) channels.Br J Pharmacol. 2009 Apr;156(8):1185-95. doi: 10.1111/j.1476-5381.2009.00111.x. Epub 2009 Mar 9. Br J Pharmacol. 2009. PMID: 19298256 Free PMC article. Review.
-
The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy.Epilepsia. 2012 Mar;53(3):412-24. doi: 10.1111/j.1528-1167.2011.03365.x. Epub 2012 Jan 5. Epilepsia. 2012. PMID: 22220513 Review.
Cited by
-
Complementary theta resonance filtering by two spatially segregated mechanisms in CA1 hippocampal pyramidal neurons.J Neurosci. 2009 Nov 18;29(46):14472-83. doi: 10.1523/JNEUROSCI.0187-09.2009. J Neurosci. 2009. PMID: 19923281 Free PMC article.
-
Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons.J Physiol. 2008 Jul 15;586(14):3493-509. doi: 10.1113/jphysiol.2008.153734. Epub 2008 May 29. J Physiol. 2008. PMID: 18511484 Free PMC article.
-
KCNQ5 channels control resting properties and release probability of a synapse.Nat Neurosci. 2011 Jun 12;14(7):840-7. doi: 10.1038/nn.2830. Nat Neurosci. 2011. PMID: 21666672 Free PMC article.
-
Bifurcation analysis of a two-compartment hippocampal pyramidal cell model.J Comput Neurosci. 2016 Aug;41(1):91-106. doi: 10.1007/s10827-016-0606-8. Epub 2016 May 25. J Comput Neurosci. 2016. PMID: 27221619 Free PMC article.
-
Antiepileptogenic and antiictogenic effects of retigabine under conditions of rapid kindling: an ontogenic study.Epilepsia. 2008 Oct;49(10):1777-86. doi: 10.1111/j.1528-1167.2008.01674.x. Epub 2008 May 21. Epilepsia. 2008. PMID: 18503560 Free PMC article.
References
-
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. - PubMed
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
-
- Amiry-Moghaddam M, Williamson A, Palomba M, Eid T, de Lanerolle NC, Nagelhus EA, Adams ME, Froehner SC, Agre P, Ottersen OP. Delayed K+ clearance associated with aquaporin-4 mislocalization: phenotypic defects in brains of alpha-syntrophin-null mice. Proc Natl Acad Sci U S A. 2003;100:13615–13620. - PMC - PubMed
-
- Andersen P, Silfvenius H, Sundberg SH, Sveen O, Wigstrom H. Functional characteristics of unmyelinated fibres in the hippocampal cortex. Brain Res. 1978;144:11–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

