Ca2+-dependent mechanisms of presynaptic control at central synapses
- PMID: 16840708
- PMCID: PMC2684670
- DOI: 10.1177/1073858405284672
Ca2+-dependent mechanisms of presynaptic control at central synapses
Abstract
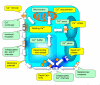
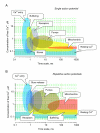
Classically, a high-power association relates the neurotransmitter release probability to the concentration of presynaptic Ca2+. Activated by the action potential waveform, voltage-gated Ca2+ channels mediate Ca2+entry into presynaptic terminals. Inside the terminal, Ca2+ ions rapidly bind to endogenous intracellular buffers and could trigger Ca2+ release from internal Ca2+ stores. The resulting space-time profile of free Ca2+ determines the time course and probability of neurotransmitter release through the interaction with molecular release triggers strategically located in the vicinity of release sites. Following a rapid concentration transient, excess Ca2+ has to be removed from the cytosol through the process involving Ca2+ uptake by the endoplasmatic reticulum stores, sequestration by mitochondria, and/or extrusion into the extracellular medium. The ongoing synaptic activity could affect any of the multiple factors that shape presynaptic Ca2+ dynamics, thus arbitrating use-dependent modification of the neurotransmitter release probability. Here we present an overview of major players involved in Ca2+-dependent presynaptic regulation of neurotransmitter release and discuss the relationships arising between their actions.
Figures

Similar articles
-
LKB1 Regulates Mitochondria-Dependent Presynaptic Calcium Clearance and Neurotransmitter Release Properties at Excitatory Synapses along Cortical Axons.PLoS Biol. 2016 Jul 18;14(7):e1002516. doi: 10.1371/journal.pbio.1002516. eCollection 2016 Jul. PLoS Biol. 2016. PMID: 27429220 Free PMC article.
-
Synaptic structural complexity as a factor enhancing probability of calcium-mediated transmitter release.J Neurophysiol. 1996 Jun;75(6):2451-66. doi: 10.1152/jn.1996.75.6.2451. J Neurophysiol. 1996. PMID: 8793756
-
[Endoplasmic reticulum and regulation of neuromediator release in presynaptic terminals].Fiziol Zh (1994). 2004;50(4):142-9. Fiziol Zh (1994). 2004. PMID: 15460039 Review. Ukrainian.
-
Presynaptic Ca2+ dynamics, Ca2+ buffers and synaptic efficacy.Cell Calcium. 2005 May;37(5):489-95. doi: 10.1016/j.ceca.2005.01.003. Cell Calcium. 2005. PMID: 15820398 Review.
-
Tracking presynaptic Ca2+ dynamics during neurotransmitter release with Ca2+-activated K+ channels.Nat Neurosci. 2000 Jun;3(6):566-71. doi: 10.1038/75737. Nat Neurosci. 2000. PMID: 10816312
Cited by
-
Neurotoxicity following acute inhalation exposure to the oil dispersant COREXIT EC9500A.J Toxicol Environ Health A. 2011;74(21):1405-18. doi: 10.1080/15287394.2011.606796. J Toxicol Environ Health A. 2011. PMID: 21916746 Free PMC article.
-
Inter-relationships among physical dimensions, distal-proximal rank orders, and basal GCaMP fluorescence levels in Ca2+ imaging of functionally distinct synaptic boutons at Drosophila neuromuscular junctions.J Neurogenet. 2018 Sep;32(3):195-208. doi: 10.1080/01677063.2018.1504043. Epub 2018 Oct 16. J Neurogenet. 2018. PMID: 30322321 Free PMC article.
-
Presynaptic K(+) channels, vesicular Ca(2+)/H (+) antiport--synaptotagmin, and acetylcholinesterase, three mechanisms cutting short the cholinergic signal at neuromuscular and nerve-electroplaque junctions.J Mol Neurosci. 2014 Jul;53(3):377-86. doi: 10.1007/s12031-013-0212-4. Epub 2014 Jan 4. J Mol Neurosci. 2014. PMID: 24390960
-
Electrophysiological studies of the effects of chronic administration of caffeine on the formation of a conditioned defensive reflex in the common snail.Neurosci Behav Physiol. 2009 May;39(4):403-7. doi: 10.1007/s11055-009-9136-4. Epub 2009 Apr 2. Neurosci Behav Physiol. 2009. PMID: 19340583
-
Involvement of NMDA receptor in the modulation of excitatory and inhibitory amino acid neurotransmitters release in cortical neurons.Neurochem Res. 2010 Sep;35(9):1478-86. doi: 10.1007/s11064-010-0209-0. Epub 2010 Jun 12. Neurochem Res. 2010. PMID: 20549556
References
-
- Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. - PubMed
-
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. - PubMed
-
- Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum. 2003;2:242–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

