A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma
- PMID: 16840727
- PMCID: PMC1895441
- DOI: 10.1182/blood-2006-04-015909
A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma
Abstract
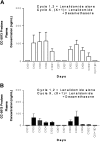
This multicenter, open-label, randomized phase 2 study evaluated 2 dose regimens of lenalidomide for relapsed, refractory myeloma. Seventy patients were randomized to receive either 30 mg once-daily or 15 mg twice-daily oral lenalidomide for 21 days of every 28-day cycle. Patients with progressive or stable disease after 2 cycles received dexamethasone. Analysis of the first 70 patients showed increased grade 3/4 myelo-suppression in patients receiving 15 mg twice daily (41% versus 13%, P = .03). An additional 32 patients received 30 mg once daily. Responses were evaluated according to European Group for Blood and Marrow Transplantation (EBMT) criteria. Overall response rate (complete, partial, or minor) to lenalidomide alone was 25% (24% for once-daily and 29% for twice-daily lenalidomide). Median overall survival in 30-mg once-daily and twice-daily groups was 28 and 27 months, respectively. Median progression-free survival was 7.7 months on once-daily versus 3.9 months on twice-daily lenalidomide (P = .2). Dexamethasone was added in 68 patients and 29% responded. Time to first occurrence of clinically significant grade 3/4 myelosuppression was shorter in the twice-daily group (1.8 vs 5.5 months, P = .05). Significant peripheral neuropathy and deep vein thrombosis each occurred in only 3%. Lenalidomide is active and well tolerated in relapsed, refractory myeloma, with the 30-mg once-daily regimen providing the basis for future studies as monotherapy and with dexamethasone.
Figures

References
-
- Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54: 8-29. - PubMed
-
- Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials: Myeloma Trialists' Collaborative Group. J Clin Oncol. 1998;16: 3832-3842. - PubMed
-
- Alexanian R, Dimopoulos M. The treatment of multiple myeloma. N Engl J Med. 1994;330: 484-489. - PubMed
-
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma: Intergroupe Francais du Myelome. N Engl J Med. 1996;335: 91-97. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348: 1875-1883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

