Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria
- PMID: 16841095
- PMCID: PMC1501111
- DOI: 10.1172/JCI27821
Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria
Abstract
The neonatal Fc receptor for IgG (FcRn) plays a major role in regulating host IgG levels and transporting IgG and associated antigens across polarized epithelial barriers. Selective expression of FcRn in the epithelium is shown here to be associated with secretion of IgG into the lumen that allows for defense against an epithelium-associated pathogen (Citrobacter rodentium). This pathway of host resistance to a bacterial pathogen as mediated by FcRn involves retrieval of bacterial antigens from the lumen and initiation of adaptive immune responses in regional lymphoid structures. Epithelial-associated FcRn, through its ability to secrete and absorb IgG, may thus integrate luminal antigen encounters with systemic immune compartments and as such provide essential host defense and immunoregulatory functions at the mucosal surfaces.
Figures

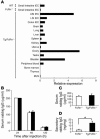

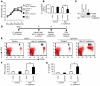
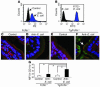
References
-
- Robert-Guroff M. IgG surfaces as an important component in mucosal protection. Nat. Med. 2000;6:129–130. - PubMed
-
- Yoshida M., et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. - PubMed
-
- Woof J.M., Mestecky J. Mucosal immunoglobulins. Immunol. Rev. 2005;206:64–82. - PubMed
-
- Hanson, L.A., and Brandzaeg, P. 1980. The mucosal defense system. InImmunologic disorders in infants and children. Stiehm, E.R., editor. W.B. Saunders. Philadelphia, Pennsylvania, USA. 137–164.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

