Population analysis of a 24-h paclitaxel infusion in advanced endometrial cancer: a gynaecological oncology group study
- PMID: 16842379
- PMCID: PMC1885077
- DOI: 10.1111/j.1365-2125.2006.02718.x
Population analysis of a 24-h paclitaxel infusion in advanced endometrial cancer: a gynaecological oncology group study
Abstract
Aims: To examine determinants of paclitaxel disposition and the association between paclitaxel exposure and toxicity or survival in patients with advanced stage or recurrent endometrial cancer treated with doxorubicin plus paclitaxel.
Methods: A limited sampling scheme was used to examine the population pharmacokinetics of paclitaxel in 160 patients from one arm of a randomized Phase III trial of doxorubicin plus paclitaxel or cisplatin. Four plasma samples per patient were collected at approximately 0, 3, 22 and 27 h after the first 24-h infusion of paclitaxel and submitted to the Gynecological Oncology Group (GOG) Pharmacology Core Laboratory. Total paclitaxel concentrations were quantified by LC/MS and paclitaxel disposition was examined using NONMEM. Paclitaxel exposure was evaluated for associations with toxicity or survival.
Results: Patient weight, age and serum glutamic-oxaloacetic transaminase level were determinants of paclitaxel clearance (clearance increased 0.437 l h-1 kg-1; decreased 0.223 l h-1 year-1 and 0.105 l h-1 IU-1). Bayesian shrinkage was minimal for this parameter. In different measures of paclitaxel exposure, AUC was most predictive of toxicity, with higher AUC associated with granulocytopenia [probability of 1% at AUC=1 to 22% at AUC=4 microg l-1 h-1 for performance status (PS)=0]. PS was more strongly associated with survival than disease stage and higher paclitaxel AUC was associated with worse survival irrespective of PS and stage.
Conclusions: Paclitaxel AUC is an independent predictor of granulocytopenia and survival in patients with advanced stage or recurrent endometrial cancer. Future studies are needed to validate the latter finding. This study confirms the appropriateness of evaluating pharmacokinetics and pharmacodynamics in multicentre oncology trials.
Figures
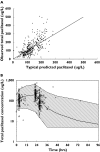
 ). (B) Ninety-five percent prediction interval (
). (B) Ninety-five percent prediction interval ( ) with the mean predicted serum concentration (
) with the mean predicted serum concentration ( ). The observed paclitaxel concentrations (○) are overlaid on the interval
). The observed paclitaxel concentrations (○) are overlaid on the interval

References
-
- Rowinsky EK, Donehower RC. Drug therapy: paclitaxel (taxol) N Engl J Med. 2003;332:1004–12. - PubMed
-
- Chan S, Gerson B. Free drug monitoring. Clin Lab Med. 1987;7:279–87. - PubMed
-
- Kumar GN, Walle UK, Bhalla KN, Walle T. Binding of taxol to human plasma, albumin and alpha 1-acid glycoprotein. Res Commun Chem Pathol Pharmacol. 1993;80:337–44. - PubMed
-
- Barre J, Didey F, Delion F, Tillement JP. Problems in therapeutic drug monitoring. Free Drug Level Monitoring. 1988;10:133–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

