Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein
- PMID: 16842382
- PMCID: PMC1885072
- DOI: 10.1111/j.1365-2125.2006.02719.x
Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein
Erratum in
- Br J Clin Pharmacol. 2010 Aug;70(2):316
Abstract
Aims: The aims of this observational study were to assess the variability in imatinib pharmacokinetics and to explore the relationship between its disposition and various biological covariates, especially plasma alpha1-acid glycoprotein concentrations.
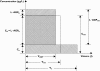
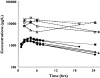
Methods: A population pharmacokinetic analysis was performed using NONMEM based on 321 plasma samples from 59 patients with either chronic myeloid leukaemia or gastrointestinal stromal tumours. The influence of covariates on oral clearance and volume of distribution was examined. Furthermore, the in vivo intracellular pharmacokinetics of imatinib was explored in five patients.
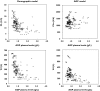
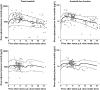
Results: A one-compartment model with first-order absorption appropriately described the data, giving a mean (+/-SEM) oral clearance of 14.3 l h-1 (+/-1.0) and a volume of distribution of 347 l (+/-62). Oral clearance was influenced by body weight, age, sex and disease diagnosis. A large proportion of the interindividual variability (36% of clearance and 63% of volume of distribution) remained unexplained by these demographic covariates. Plasma alpha1-acid glycoprotein concentrations had a marked influence on total imatinib concentrations. Moreover, we observed an intra/extracellular ratio of 8, suggesting substantial uptake of the drug into the target cells.
Conclusion: Because of the high pharmacokinetic variability of imatinib and the reported relationships between its plasma concentration and efficacy and toxicity, the usefulness of therapeutic drug monitoring as an aid to optimizing therapy should be further investigated. Ideally, such an approach should take account of either circulating alpha1-acid glycoprotein concentrations or free imatinib concentrations.
Figures
Similar articles
-
Population pharmacokinetics of imatinib mesylate and its metabolite in children and young adults.Cancer Chemother Pharmacol. 2009 Jan;63(2):229-38. doi: 10.1007/s00280-008-0730-x. Epub 2008 Apr 9. Cancer Chemother Pharmacol. 2009. PMID: 18398615 Clinical Trial.
-
Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors.Clin Cancer Res. 2006 Oct 15;12(20 Pt 1):6073-8. doi: 10.1158/1078-0432.CCR-05-2596. Clin Cancer Res. 2006. PMID: 17062683
-
Alpha1 acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients.Clin Cancer Res. 2003 Feb;9(2):625-32. Clin Cancer Res. 2003. PMID: 12576428 Clinical Trial.
-
The role of therapeutic drug monitoring of imatinib in patients with chronic myeloid leukemia and metastatic or unresectable gastrointestinal stromal tumors.Ther Drug Monit. 2012 Feb;34(1):85-97. doi: 10.1097/FTD.0b013e31823cdec9. Ther Drug Monit. 2012. PMID: 22215488 Review.
-
Clinical pharmacokinetics of imatinib mesylate.In Vivo. 2005 Jan-Feb;19(1):77-84. In Vivo. 2005. PMID: 15796158 Review.
Cited by
-
Human hepatocyte assessment of imatinib drug-drug interactions - complexities in clinical translation.Br J Clin Pharmacol. 2015 Nov;80(5):1097-108. doi: 10.1111/bcp.12723. Epub 2015 Sep 19. Br J Clin Pharmacol. 2015. PMID: 26178713 Free PMC article.
-
Tyrosine kinase inhibitors in the treatment of systemic sclerosis: from animal models to clinical trials.Curr Rheumatol Rep. 2011 Feb;13(1):21-7. doi: 10.1007/s11926-010-0142-x. Curr Rheumatol Rep. 2011. PMID: 21042889 Review.
-
Relationship of imatinib-free plasma levels and target genotype with efficacy and tolerability.Br J Cancer. 2008 May 20;98(10):1633-40. doi: 10.1038/sj.bjc.6604355. Epub 2008 May 6. Br J Cancer. 2008. PMID: 18475296 Free PMC article.
-
Pharmacokinetically based estimation of patient compliance with oral anticancer chemotherapies: in silico evaluation.Clin Pharmacokinet. 2009;48(6):359-69. doi: 10.2165/00003088-200948060-00002. Clin Pharmacokinet. 2009. PMID: 19650675
-
Predictive performance of population pharmacokinetic models of imatinib in chronic myeloid leukemia patients.Cancer Chemother Pharmacol. 2024 Jul;94(1):35-44. doi: 10.1007/s00280-024-04644-w. Epub 2024 Mar 5. Cancer Chemother Pharmacol. 2024. PMID: 38441626 Free PMC article.
References
-
- Druker BJ. Imatinib mesylate in the treatment of chronic myeloid leukaemia. Expert Opin Pharmacother. 2003;4:963–71. - PubMed
-
- Tothova E, Kafkova A, Fricova M, Benova B, Kirschnerova G, Tothova A. Imatinib mesylate in Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon alpha. Neoplasma. 2005;52:63–7. - PubMed
-
- Steinert DM, McAuliffe JC, Trent JC. Imatinib mesylate in the treatment of gastrointestinal stromal tumour. Expert Opin Pharmacother. 2005;6:105–13. - PubMed
-
- Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502. - PubMed
-
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

