The in vitro metabolism of irinotecan (CPT-11) by carboxylesterase and beta-glucuronidase in human colorectal tumours
- PMID: 16842384
- PMCID: PMC1885078
- DOI: 10.1111/j.1365-2125.2005.02477.x
The in vitro metabolism of irinotecan (CPT-11) by carboxylesterase and beta-glucuronidase in human colorectal tumours
Abstract
Aims: Irinotecan (CPT-11) is a prodrug that is used to treat metastatic colorectal cancer. It is activated to the topoisomerase poison SN-38 by carboxylesterases. SN-38 is metabolized to its inactive glucuronide, SN-38 glucuronide. The aim of this study was to determine, the reactivation of SN-38 from SN-38 glucuronide by beta-glucuronidase may represent a significant pathway of SN-38 formation.
Methods: The production of SN-38 from irinotecan and SN-38 glucuronide (2.4, 9.6 and 19.2 microm) was measured in homogenates of human colorectal tumour, and matched normal colon mucosa from 21 patients).
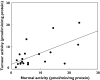
Results: The rate of conversion of irinotecan (9.6 microm) was lower in tumour tissue than matched normal colon mucosa samples (0.30+/-0.14 pmol min-1 mg-1 protein and 0.77+/-0.59 pmol min-1 mg-1 protein, respectively; P<0.005). In contrast, no significant difference was observed in beta-glucuronidase activity between tumour and matched normal colon samples (4.56+/-6.9 pmol min-1 mg-1 protein and 3.62+/-2.95 pmol min-1 mg-1 protein, respectively, using 9.6 microm SN-38 glucuronide; P>0.05). beta-Glucuronidase activity in tumour correlated to that observed in matched normal tissue (r2>0.23, P<0.05), whereas this was not the case for carboxylesterase activity. At equal concentrations of irinotecan and SN-38 glucuronide, the rate of beta-glucuronidase-mediated SN-38 production was higher than that formed from irinotecan in both tumour and normal tissue (P<0.05). However, at concentrations that reflect the relative plasma concentrations observed in patients, the rate of SN-38 production via these two pathways was comparable.
Conclusions: Tumour beta-glucuronidase may play a significant role in the exposure of tumours to SN-38 in vivo.
Figures
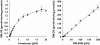
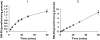



Similar articles
-
The relative contributions of carboxylesterase and beta-glucuronidase in the formation of SN-38 in human colorectal tumours.Oncol Rep. 2003 Nov-Dec;10(6):1977-9. Oncol Rep. 2003. PMID: 14534729
-
The importance of tumor glucuronidase in the activation of irinotecan in a mouse xenograft model.J Pharmacol Exp Ther. 2002 Nov;303(2):649-55. doi: 10.1124/jpet.102.039040. J Pharmacol Exp Ther. 2002. PMID: 12388647
-
Local enzymatic hydrolysis of an endogenously generated metabolite can enhance CPT-11 anticancer efficacy.Mol Cancer Ther. 2009 Apr;8(4):940-6. doi: 10.1158/1535-7163.MCT-08-0812. Mol Cancer Ther. 2009. PMID: 19372567
-
Pharmacology of irinotecan.Oncology (Williston Park). 1998 Aug;12(8 Suppl 6):39-42. Oncology (Williston Park). 1998. PMID: 9726089 Review.
-
Metabolism of CPT-11. Impact on activity.Ann N Y Acad Sci. 2000;922:205-15. doi: 10.1111/j.1749-6632.2000.tb07039.x. Ann N Y Acad Sci. 2000. PMID: 11193896 Review.
Cited by
-
Intra-arterial Therapy Using Micellar Nanoparticles Incorporating SN-38 in a Rat Pancreatic Tumor Model.Cardiovasc Intervent Radiol. 2025 Mar;48(3):372-378. doi: 10.1007/s00270-024-03939-y. Epub 2025 Feb 21. Cardiovasc Intervent Radiol. 2025. PMID: 39984668
-
Thiazolidin-2-cyanamides derivatives as novel potent Escherichia coli β-glucuronidase inhibitors and their structure-inhibitory activity relationships.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1736-1742. doi: 10.1080/14756366.2020.1816998. J Enzyme Inhib Med Chem. 2020. PMID: 32928007 Free PMC article.
-
Does Tumour Contrast Retention on CT Immediately Post Chemoembolization Predict Tumour Metabolic Response on FDG-PET in Patients with Hepatic Metastases from Colorectal Cancer?Gastroenterol Res Pract. 2019 Nov 4;2019:7279163. doi: 10.1155/2019/7279163. eCollection 2019. Gastroenterol Res Pract. 2019. PMID: 31781199 Free PMC article.
-
Photodynamic Therapy Synergizes with Irinotecan to Overcome Compensatory Mechanisms and Improve Treatment Outcomes in Pancreatic Cancer.Cancer Res. 2016 Mar 1;76(5):1066-77. doi: 10.1158/0008-5472.CAN-15-0391. Epub 2015 Dec 30. Cancer Res. 2016. PMID: 26719532 Free PMC article.
-
Potential repurposing of known drugs as potent bacterial β-glucuronidase inhibitors.J Biomol Screen. 2012 Aug;17(7):957-65. doi: 10.1177/1087057112444927. Epub 2012 Apr 24. J Biomol Screen. 2012. PMID: 22535688 Free PMC article.
References
-
- Gupta E, Lestingi TM, Mick R, Ramirez J, Vokes EE, Ratain MJ. Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res. 1994;54:3723–5. - PubMed
-
- Haaz MC, Rivory LP, Riche C, Robert J. The transformation of irinotecan (CPT-11) to its active metabolite SN- 38 by human liver microsomes. Differential hydrolysis for the lactone and carboxylate forms. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:257–62. - PubMed
-
- Rivory LP, Bowles MR, Robert J, Pond SM. Conversion of irinotecan (CPT-11) to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by human liver carboxylesterase. Biochem Pharmacol. 1996;52:1103–11. - PubMed
-
- Satoh T, Hosokawa M, Atsumi R, Suzuki W, Hakusui H, Nagai E. Metabolic activation of CPT-11, 7-ethyl-10-[4-(1-piperidino)-1-piperidino]carbonyloxycamptothecin, a novel antitumor agent, by carboxylesterase. Biol Pharm Bull. 1994;17:662–4. - PubMed
-
- Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

