Poor correlation between 6beta-hydroxycortisol:cortisol molar ratios and midazolam clearance as measure of hepatic CYP3A activity
- PMID: 16842393
- PMCID: PMC1885097
- DOI: 10.1111/j.1365-2125.2006.02628.x
Poor correlation between 6beta-hydroxycortisol:cortisol molar ratios and midazolam clearance as measure of hepatic CYP3A activity
Abstract
Aims: A non-invasive proposed method for measuring CYP3A activity is the urinary 6beta-hydroxycortisol:cortisol ratio. This ratio has been used as an indicator of CYP3A induction and inhibition, with mixed results. This investigation evaluated the relationship between a validated, biomarker, intravenous midazolam clearance and the urinary cortisol ratio under constitutive conditions and with the influence of a moderate CYP3A inhibitor.
Methods: This was a sequential, cross-over study design. Intravenous midazolam 0.025 mg kg(-1) was administered to 10 male and 10 female subjects once every 14 days for 4 months. Fluvoxamine 150 mg day(-1) was given to all subjects during the last two visits. Total body clearance of midazolam and urinary 6beta-hydroxycortisol:cortisol molar ratio were used as biomarkers of hepatic CYP3A activity.
Results: No significant correlations were found between these two markers (r(2) < 0.5, P > 0.05). Larger interindividual and intra-individual variability in CYP3A activity was observed in 6beta-hydroxycortisol:cortisol ratios compared with midazolam clearances. With fluvoxamine therapy, midazolam clearance values decreased approximately 1.5-fold and cortisol ratios decreased approximately 1.9-fold.
Conclusions: The high intra-individual variability of the urinary cortisol ratio, compared with midazolam, makes this a suboptimal CYP3A phenotyping tool.
Figures
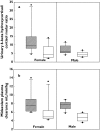
 ) post treatment (□)
) post treatment (□)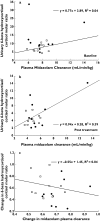
Comment in
-
Poor correlation between 6beta-hydroxycortisol:cortisol molar ratios and midazolam clearance as measure of hepatic CYP3A activity: a comment.Br J Clin Pharmacol. 2007 May;63(5):632; author reply 633. doi: 10.1111/j.1365-2125.2006.02804.x. Epub 2006 Nov 10. Br J Clin Pharmacol. 2007. PMID: 17094779 Free PMC article. No abstract available.
Similar articles
-
Urinary 6β-Hydroxycortisol/Cortisol Ratio Most Highly Correlates With Midazolam Clearance Under Hepatic CYP3A Inhibition and Induction in Females: A Pharmacometabolomics Approach.AAPS J. 2016 Sep;18(5):1254-1261. doi: 10.1208/s12248-016-9941-y. Epub 2016 Jun 17. AAPS J. 2016. PMID: 27317471 Clinical Trial.
-
Associations of the CYP3A5*3 and CYP3A4*1G polymorphisms with the pharmacokinetics of oral midazolam and the urinary 6β-hydroxycortisol/cortisol ratio as markers of CYP3A activity in healthy male Chinese.J Clin Pharm Ther. 2016 Oct;41(5):552-8. doi: 10.1111/jcpt.12433. Epub 2016 Aug 11. J Clin Pharm Ther. 2016. PMID: 27511886
-
Poor correlation between 6beta-hydroxycortisol:cortisol molar ratios and midazolam clearance as measure of hepatic CYP3A activity: a comment.Br J Clin Pharmacol. 2007 May;63(5):632; author reply 633. doi: 10.1111/j.1365-2125.2006.02804.x. Epub 2006 Nov 10. Br J Clin Pharmacol. 2007. PMID: 17094779 Free PMC article. No abstract available.
-
Urinary 6beta-hydroxycortisol: a validated test for evaluating drug induction or drug inhibition mediated through CYP3A in humans and in animals.Eur J Clin Pharmacol. 2003 Dec;59(10):713-33. doi: 10.1007/s00228-003-0690-3. Epub 2003 Nov 6. Eur J Clin Pharmacol. 2003. PMID: 14605790 Review.
-
Perspective: 4β-hydroxycholesterol as an emerging endogenous biomarker of hepatic CYP3A.Drug Metab Rev. 2017 Feb;49(1):18-34. doi: 10.1080/03602532.2016.1239630. Epub 2016 Oct 20. Drug Metab Rev. 2017. PMID: 27718639 Review.
Cited by
-
The Role of CYP3A in Health and Disease.Biomedicines. 2022 Oct 24;10(11):2686. doi: 10.3390/biomedicines10112686. Biomedicines. 2022. PMID: 36359206 Free PMC article. Review.
-
Novel Approaches to Characterize Individual Drug Metabolism and Advance Precision Medicine.Drug Metab Dispos. 2023 Oct;51(10):1238-1253. doi: 10.1124/dmd.122.001066. Epub 2023 Jul 7. Drug Metab Dispos. 2023. PMID: 37419681 Free PMC article. Review.
-
Identification of the caffeine to trimethyluric acid ratio as a dietary biomarker to characterise variability in cytochrome P450 3A activity.Eur J Clin Pharmacol. 2019 Sep;75(9):1211-1218. doi: 10.1007/s00228-019-02682-5. Epub 2019 May 23. Eur J Clin Pharmacol. 2019. PMID: 31123759 Clinical Trial.
-
Interindividual Variability in Cytochrome P450-Mediated Drug Metabolism.Drug Metab Dispos. 2016 Mar;44(3):343-51. doi: 10.1124/dmd.115.067900. Epub 2015 Dec 17. Drug Metab Dispos. 2016. PMID: 26681736 Free PMC article. Review.
-
A Physiology-Based Pharmacokinetic Framework to Support Drug Development and Dose Precision During Therapeutic Hypothermia in Neonates.Front Pharmacol. 2020 May 13;11:587. doi: 10.3389/fphar.2020.00587. eCollection 2020. Front Pharmacol. 2020. PMID: 32477113 Free PMC article. Review.
References
-
- Slaughter RL, Edwards DJ. Recent advances: the cytochrome P450 enzymes. Ann Pharmacother. 1995;29:619–24. - PubMed
-
- Thummel KE, Shen DD, Podoll TD, Kunze KL, Trager WF, Bacchi CE, Marsh CL, McVicar JP, Barr DM, Perkins JD, et al. Use of midazolam as a human cytochrome P4503A probe; II. Characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Ther. 1994;27:557–66. - PubMed
-
- Dowling TC, Briglia AE, Fink JC, Hanes DS, Light PD, Stackiewicz L, Karyekar CS, Eddington ND, Weir MR, Henrich WL. Characterization of hepatic cytochrome p4503A activity in patients with end-stage renal disease. Clin Pharmacol Ther. 2003;73:427–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

