Multiple doses of the antimuscarinic agent solifenacin do not affect the pharmacodynamics or pharmacokinetics of warfarin or the steady-state pharmacokinetics of digoxin in healthy subjects
- PMID: 16842396
- PMCID: PMC1885095
- DOI: 10.1111/j.1365-2125.2006.02638.x
Multiple doses of the antimuscarinic agent solifenacin do not affect the pharmacodynamics or pharmacokinetics of warfarin or the steady-state pharmacokinetics of digoxin in healthy subjects
Abstract
Aims: Solifenacin succinate is used for the treatment of overactive bladder (OAB). The potential for pharmacokinetic and/or pharmacodynamic interactions between solifenacin and warfarin or digoxin was investigated.
Methods: The solifenacin-warfarin study was a two-period crossover trial conducted in healthy males. Subjects received warfarin on the 10th day of 16 days of dosing with either solifenacin or placebo. The solifenacin-digoxin study was an one-sequence crossover trial conducted in healthy males and females. Following a phase-in period for digoxin, solifenacin was administered concomitantly with the drug on days 9-18.
Results: The AUC(PT; 0-168 h) following a single dose of warfarin was unchanged in the presence of solifenacin [point estimate = 1.005; 90% confidence interval (CI) 0.98, 1.02)]. The AUC(0-infinity) values for both warfarin enantiomers were also unchanged. A small increase in the C(max) of digoxin was observed during treatment with solifenacin, but for AUC(ss,tau) and C(max) the 90% CI fell within the prespecified interval of 0.80-1.25. Combined administration of solifenacin and warfarin or digoxin was well tolerated.
Conclusions: Since the pharmacokinetics and pharmacodynamics of a single dose of warfarin and the steady-state pharmacokinetics of digoxin were not affected by coadministration of solifenacin in healthy subjects, the need for dosing adjustments for digoxin and/or warfarin does not seem warranted.
Figures
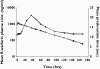
 ), warfarin without solifenacin (▪), and prothrombin time, warfarin with solifenacin (
), warfarin without solifenacin (▪), and prothrombin time, warfarin with solifenacin ( ), warfarin without solifenacin (▴) vs. time profiles of R-warfarin in the absence and presence of solifenacin
), warfarin without solifenacin (▴) vs. time profiles of R-warfarin in the absence and presence of solifenacin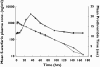
 ), warfarin without solifenacin (
), warfarin without solifenacin ( ), and prothrombin time, warfarin with solifenacin (♦), warfarin without solifenacin (▴) vs. profiles of S-warfarin in the absence and presence of solifenacin
), and prothrombin time, warfarin with solifenacin (♦), warfarin without solifenacin (▴) vs. profiles of S-warfarin in the absence and presence of solifenacin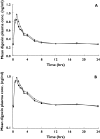
 ), digoxin with solifenacin (
), digoxin with solifenacin ( )
)Similar articles
-
Effect of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on the pharmacokinetics of oral contraceptives, warfarin, and digoxin in healthy participants.Int J Clin Pharmacol Ther. 2015 Jan;53(1):41-53. doi: 10.5414/CP202157. Int J Clin Pharmacol Ther. 2015. PMID: 25345427 Clinical Trial.
-
Pharmacokinetic effect of ketoconazole on solifenacin in healthy volunteers.Basic Clin Pharmacol Toxicol. 2006 Jul;99(1):33-6. doi: 10.1111/j.1742-7843.2006.pto_285.x. Basic Clin Pharmacol Toxicol. 2006. PMID: 16867168 Clinical Trial.
-
Pharmacokinetics and safety of solifenacin succinate in healthy young men.J Clin Pharmacol. 2004 Sep;44(9):1023-33. doi: 10.1177/0091270004267592. J Clin Pharmacol. 2004. PMID: 15317830 Clinical Trial.
-
Solifenacin significantly improves all symptoms of overactive bladder syndrome.Int J Clin Pract. 2006 Aug;60(8):959-66. doi: 10.1111/j.1742-1241.2006.01067.x. Int J Clin Pract. 2006. PMID: 16893438 Free PMC article. Review.
-
Solifenacin succinate for the treatment of overactive bladder.Expert Opin Drug Metab Toxicol. 2009 Mar;5(3):345-50. doi: 10.1517/17425250902762866. Expert Opin Drug Metab Toxicol. 2009. PMID: 19331596 Review.
Cited by
-
Clinical pharmacokinetics and pharmacodynamics of solifenacin.Clin Pharmacokinet. 2009;48(5):281-302. doi: 10.2165/00003088-200948050-00001. Clin Pharmacokinet. 2009. PMID: 19566112 Review.
References
-
- McGhan WF. Cost effectiveness and quality of life considerations in the treatment of patients with overactive bladder. Am J Manag Care. 2001;7(2 Suppl.):S62–75. - PubMed
-
- Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder. Urol. 2002;60(Suppl. 5A):7–12. - PubMed
-
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Kerrebroeck P, Victor A, Wein A. The standardisation of terminology in lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Urology. 2003;61:37–49. - PubMed
-
- Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760–6. - PubMed
-
- Versi E. Screening initiative confirms widespread prevalence of overactive bladder in American adults. Int Urogynecol J. 2001;12:S13.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

