Notch signaling plays a key role in cardiac cell differentiation
- PMID: 16843648
- PMCID: PMC1567976
- DOI: 10.1016/j.mod.2006.06.003
Notch signaling plays a key role in cardiac cell differentiation
Abstract
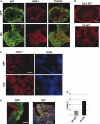
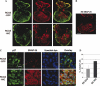
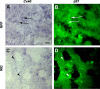
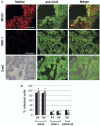
Results from lineage tracing studies indicate that precursor cells in the ventricles give rise to both cardiac muscle and conduction cells. Cardiac conduction cells are specialized cells responsible for orchestrating the rhythmic contractions of the heart. Here, we show that Notch signaling plays an important role in the differentiation of cardiac muscle and conduction cell lineages in the ventricles. Notch1 expression coincides with a conduction marker, HNK-1, at early stages. Misexpression of constitutively active Notch1 (NIC) in early heart tubes in chick exhibited multiple effects on cardiac cell differentiation. Cells expressing NIC had a significant decrease in expression of cardiac muscle markers, but an increase in expression of conduction cell markers, HNK-1, and SNAP-25. However, the expression of the conduction marker connexin 40 was inhibited. Loss-of-function study, using a dominant-negative form of Suppressor-of-Hairless, further supports that Notch1 signaling is important for the differentiation of these cardiac cell types. Functional studies show that the expression of constitutively active Notch1 resulted in abnormalities in ventricular conduction pathway patterns.
Figures



Similar articles
-
Activated Notch1 alters differentiation of embryonic stem cells into mesodermal cell lineages at multiple stages of development.Mech Dev. 2006 Jul;123(7):570-9. doi: 10.1016/j.mod.2006.05.002. Epub 2006 May 25. Mech Dev. 2006. PMID: 16822655
-
Activation of Notch1 signaling in cardiogenic mesoderm induces abnormal heart morphogenesis in mouse.Development. 2006 May;133(9):1625-34. doi: 10.1242/dev.02344. Epub 2006 Mar 22. Development. 2006. PMID: 16554359
-
Notch1b and neuregulin are required for specification of central cardiac conduction tissue.Development. 2006 Mar;133(6):1125-32. doi: 10.1242/dev.02279. Epub 2006 Feb 15. Development. 2006. PMID: 16481353
-
Notch signaling as an important mediator of cardiac repair and regeneration after myocardial infarction.Trends Cardiovasc Med. 2010 Oct;20(7):228-31. doi: 10.1016/j.tcm.2011.11.006. Trends Cardiovasc Med. 2010. PMID: 22293023 Free PMC article. Review.
-
Developmental Origin of the Cardiac Conduction System: Insight from Lineage Tracing.Pediatr Cardiol. 2018 Aug;39(6):1107-1114. doi: 10.1007/s00246-018-1906-8. Epub 2018 May 17. Pediatr Cardiol. 2018. PMID: 29774393 Free PMC article. Review.
Cited by
-
Optical Mapping of Cardiomyocytes in Monolayer Derived from Induced Pluripotent Stem Cells.Cells. 2023 Aug 29;12(17):2168. doi: 10.3390/cells12172168. Cells. 2023. PMID: 37681899 Free PMC article. Review.
-
Notch Intracellular Domain Plasmid Delivery via Poly(Lactic-Co-Glycolic Acid) Nanoparticles to Upregulate Notch Pathway Molecules.Front Cardiovasc Med. 2021 Sep 28;8:707897. doi: 10.3389/fcvm.2021.707897. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34651022 Free PMC article.
-
MicroRNA-375 overexpression influences P19 cell proliferation, apoptosis and differentiation through the Notch signaling pathway.Int J Mol Med. 2016 Jan;37(1):47-55. doi: 10.3892/ijmm.2015.2399. Epub 2015 Nov 2. Int J Mol Med. 2016. PMID: 26531318 Free PMC article.
-
Modeling Cardiac Disease Mechanisms Using Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Progress, Promises and Challenges.Int J Mol Sci. 2020 Jun 19;21(12):4354. doi: 10.3390/ijms21124354. Int J Mol Sci. 2020. PMID: 32575374 Free PMC article. Review.
-
Cardiomyogenic stem and progenitor cell plasticity and the dissection of cardiopoiesis.J Mol Cell Cardiol. 2008 Oct;45(4):475-94. doi: 10.1016/j.yjmcc.2008.05.002. Epub 2008 May 11. J Mol Cell Cardiol. 2008. PMID: 18565538 Free PMC article. Review.
References
-
- Aoyama N, Kikawada R, Yamashina S. Immunohistochemical study on the development of the rat heart conduction system using anti-Leu-7 antibody. Arch. Histol. Cytol. 1993;56:303–315. - PubMed
-
- Aoyama N, Tamaki H, Kikawada R, Yamashina S. Development of the conduction system in the rat heart as determined by Leu-7 (HNK-1) immunohistochemistry and computer graphics reconstruction. Lab. Invest. 1995;72:355–366. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Bao ZZ, Bruneau BG, Seidman JG, Seidman CE, Cepko CL. Regulation of chamber-specific gene expression in the developing heart by Irx4. Science. 1999;283:1161–1164. - PubMed
-
- Bastide B, Neyses L, Ganten D, Paul M, Willecke K, Traub O. Gap junction protein connexin40 is preferentially expressed in vascular endothelium and conductive bundles of rat myocardium and is increased under hypertensive conditions. Circ. Res. 1993;73:1138–1149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

