Structure-function relationship in a variant hemoglobin: a combined computational-experimental approach
- PMID: 16844744
- PMCID: PMC1614504
- DOI: 10.1529/biophysj.106.083170
Structure-function relationship in a variant hemoglobin: a combined computational-experimental approach
Abstract
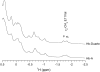
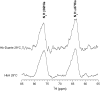
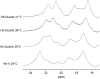
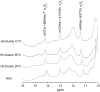
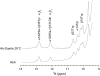
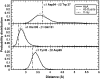
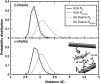
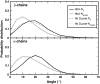
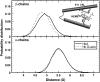
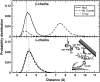
Our study examines the functional and structural effects of amino acid substitution in the distal side of beta-chains of human Hb Duarte (alpha(2)beta(2)(62Ala-->Pro)). We have compared the functional properties of the purified Hb Duarte with those of HbA, and through proton NMR and molecular dynamics simulations we have investigated their tertiary and quaternary structures. The variant exhibits an increased oxygen affinity with a normal Hill coefficient and Bohr effect. The abnormal function of Hb Duarte is attributed to the presence of a proline residue at the beta62 position, since the functional properties of another Hb variant in the same position, Hb J-Europa (beta(62Ala-->Asp)), have been described as normal. Thereafter (1)H-NMR studies have shown that the beta62 Ala-->Pro substitution causes structural modifications of the tertiary structure of the beta globins, leaving the quaternary structure unaltered. These results have been confirmed by extensive all-atom molecular dynamics simulations. All these findings lead to the conclusion that the beta62 Ala-->Pro substitution produces a destabilization of the E-helix extending downward to the CD corner. Particularly, a cavity near the distal histidine of the beta-chains, connecting the heme pocket to the solvent, is affected, altering the functional properties of the protein molecule.
Figures





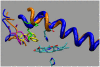

Similar articles
-
Ligand binding properties and structural studies of recombinant and chemically modified hemoglobins altered at beta 93 cysteine.Biochemistry. 2002 Oct 1;41(39):11901-13. doi: 10.1021/bi0202880. Biochemistry. 2002. PMID: 12269835
-
Effects of substitutions of lysine and aspartic acid for asparagine at beta 108 and of tryptophan for valine at alpha 96 on the structural and functional properties of human normal adult hemoglobin: roles of alpha 1 beta 1 and alpha 1 beta 2 subunit interfaces in the cooperative oxygenation process.Biochemistry. 1999 Jul 6;38(27):8751-61. doi: 10.1021/bi990286o. Biochemistry. 1999. PMID: 10393550
-
Structural and functional studies of hemoglobin Poissy alpha 2 beta 2(56) (D7) Gly----Arg and 86 (F2) Ala----Pro.Eur J Biochem. 1985 Dec 16;153(3):655-62. doi: 10.1111/j.1432-1033.1985.tb09350.x. Eur J Biochem. 1985. PMID: 3841063
-
Restoring allosterism with compensatory mutations in hemoglobin.Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11547-51. doi: 10.1073/pnas.91.24.11547. Proc Natl Acad Sci U S A. 1994. PMID: 7972099 Free PMC article.
-
Structural and Functional Characterization of a New Double Variant Haemoglobin (HbG-Philadelphia/Duarte α(2)β(2)).ISRN Hematol. 2011;2011:735314. doi: 10.5402/2011/735314. Epub 2010 Nov 29. ISRN Hematol. 2011. PMID: 22084702 Free PMC article.
Cited by
-
Molecular dynamics simulation of a carboxy murine neuroglobin mutated on the proximal side: heme displacement and concomitant rearrangement in loop regions.J Mol Model. 2010 Apr;16(4):759-70. doi: 10.1007/s00894-009-0581-3. Epub 2009 Oct 8. J Mol Model. 2010. PMID: 19820972
-
Haemoglobin polymorphisms affect the oxygen-binding properties in Atlantic cod populations.Proc Biol Sci. 2009 Mar 7;276(1658):833-41. doi: 10.1098/rspb.2008.1529. Proc Biol Sci. 2009. PMID: 19033139 Free PMC article.
-
Dynamic features of carboxy cytoglobin distal mutants investigated by molecular dynamics simulations.J Biol Inorg Chem. 2016 Apr;21(2):251-61. doi: 10.1007/s00775-016-1334-2. Epub 2016 Feb 3. J Biol Inorg Chem. 2016. PMID: 26841790
-
Dynamics of camel and human hemoglobin revealed by molecular simulations.Sci Rep. 2022 Jan 7;12(1):122. doi: 10.1038/s41598-021-04112-y. Sci Rep. 2022. PMID: 34997093 Free PMC article.
-
Effects of distal mutation on the dynamic properties of carboxycytoglobin: a molecular dynamics simulation study.J Biol Inorg Chem. 2013 Dec;18(8):947-55. doi: 10.1007/s00775-013-1041-1. Epub 2013 Sep 14. J Biol Inorg Chem. 2013. PMID: 24037220
References
-
- Lukin, J. A., and C. Ho. 2004. The structure-function relationship of hemoglobin in solution at atomic resolution. Chem. Rev. 104:1219–1230. - PubMed
-
- Perutz, M. F. 1968. Preparation of haemoglobin crystals. J. Cryst. Growth. 2:54–56.
-
- Perutz, M. F., G. Fermi, B. Luisi, B. Shaanan, and R. C. Liddington. 1987. Stereochemistry of cooperativity mechanisms in hemoglobin. Acc. Chem. Res. 20:309–321. - PubMed
-
- Vandegriff, K. D., A. Malavalli, J. Wooldridge, J. Lohman, and R. M. Winslow. 2003. MP4, a new nonvasoactive PEG-Hb conjugate. Transfusion. 43:509–516. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

