Body temperature-related structural transitions of monotremal and human hemoglobin
- PMID: 16844747
- PMCID: PMC1578488
- DOI: 10.1529/biophysj.106.087809
Body temperature-related structural transitions of monotremal and human hemoglobin
Abstract
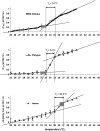
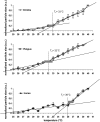
In this study, temperature-related structural changes were investigated in human, duck-billed platypus (Ornithorhynchus anatinus, body temperature T(b) = 31-33 degrees C), and echidna (Tachyglossus aculeatus, body temperature T(b) = 32-33 degrees C) hemoglobin using circular dichroism spectroscopy and dynamic light scattering. The average hydrodynamic radius (R(h)) and fractional (normalized) change in the ellipticity (F(obs)) at 222 +/- 2 nm of hemoglobin were measured. The temperature was varied stepwise from 25 degrees C to 45 degrees C. The existence of a structural transition of human hemoglobin at the critical temperature T(c) between 36-37 degrees C was previously shown by micropipette aspiration experiments, viscosimetry, and circular dichroism spectroscopy. Based on light-scattering measurements, this study proves the onset of molecular aggregation at T(c). In two different monotremal hemoglobins (echidna and platypus), the critical transition temperatures were found between 32-33 degrees C, which are close to the species' body temperature T(b). The data suggest that the correlation of the structural transition's critical temperature T(c) and the species' body temperature T(b) is not mere coincidence but, instead, is a more widespread structural phenomenon possibly including many other proteins.
Figures


Similar articles
-
Structural transition temperature of hemoglobins correlates with species' body temperature.Eur Biophys J. 2007 Dec;37(1):1-10. doi: 10.1007/s00249-007-0144-4. Epub 2007 Mar 28. Eur Biophys J. 2007. PMID: 17390129
-
Distinct development of peripheral trigeminal pathways in the platypus (Ornithorhynchus anatinus) and short-beaked echidna (Tachyglossus aculeatus).Brain Behav Evol. 2012;79(2):113-27. doi: 10.1159/000334469. Epub 2011 Dec 16. Brain Behav Evol. 2012. PMID: 22179203
-
Iron(III) binding proteins of echidna (Tachyglossus aculeatus) and platypus (Ornithorhynchus anatinus).Biochem Int. 1990 Oct;22(2):321-8. Biochem Int. 1990. PMID: 2090097
-
Sensory receptors in monotremes.Philos Trans R Soc Lond B Biol Sci. 1998 Jul 29;353(1372):1187-98. doi: 10.1098/rstb.1998.0275. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9720114 Free PMC article. Review.
-
Field biology of the platypus (Ornithorhynchus anatinus): historical and current perspectives.Philos Trans R Soc Lond B Biol Sci. 1998 Jul 29;353(1372):1081-91. doi: 10.1098/rstb.1998.0267. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9720106 Free PMC article. Review.
Cited by
-
Effects of spermine NONOate and ATP on the thermal stability of hemoglobin.BMC Biophys. 2012 Aug 28;5:16. doi: 10.1186/2046-1682-5-16. BMC Biophys. 2012. PMID: 22929146 Free PMC article.
-
Sequential events in the irreversible thermal denaturation of human brain-type creatine kinase by spectroscopic methods.Int J Mol Sci. 2010 Jun 25;11(7):2584-96. doi: 10.3390/ijms11072584. Int J Mol Sci. 2010. PMID: 20717523 Free PMC article.
-
Oncological hyperthermia: The correct dosing in clinical applications.Int J Oncol. 2019 Feb;54(2):627-643. doi: 10.3892/ijo.2018.4645. Epub 2018 Nov 23. Int J Oncol. 2019. PMID: 30483754 Free PMC article.
-
Size Sensitivity of Metabolite Diffusion in Macromolecular Crowds.Nano Lett. 2024 Apr 12;24(16):4801-9. doi: 10.1021/acs.nanolett.3c05100. Online ahead of print. Nano Lett. 2024. PMID: 38607288 Free PMC article.
-
Molecular rotors in haemoglobin and bovine serum albumin proteins.J R Soc Interface. 2022 Nov;19(196):20220709. doi: 10.1098/rsif.2022.0709. Epub 2022 Nov 30. J R Soc Interface. 2022. PMID: 36448286 Free PMC article.
References
-
- Bettati, S., A. Mozzarelli, and M. F. Perutz. 1998. Allosteric mechanism of haemoglobin: rupture of salt-bridges raises the oxygen affinity of the T-structure. J. Mol. Biol. 281:581–585. - PubMed
-
- Knapp, J. E., M. A. Oliveira, Q. Xie, S. R. Ernst, A. F. Riggs, and M. L. Hackert. 1999. The structural and functional analysis of the hemoglobin D component from chicken. J. Biol. Chem. 274:6411–6420. - PubMed
-
- Levantino, M., A. Cupane, and L. Zimanyi. 2003. Quaternary structure dependence of kinetic hole burning and conformational substates interconversion in hemoglobin. Biochemistry. 42:4499–4505. - PubMed
-
- Bischof, J. C., and X. He. 2006. Thermal stability of proteins. Ann. N. Y. Acad. Sci. 1066:12–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

