Kinesin's biased stepping mechanism: amplification of neck linker zippering
- PMID: 16844749
- PMCID: PMC1562392
- DOI: 10.1529/biophysj.106.087049
Kinesin's biased stepping mechanism: amplification of neck linker zippering
Abstract
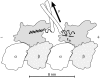
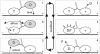
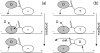
A physically motivated model of kinesin's motor function is developed within the framework of rectified Brownian motion. The model explains how the amplification of neck linker zippering arises naturally through well-known formulae for overdamped dynamics, thereby providing a means to understand how weakly-favorable zippering leads to strongly favorable plus-directed binding of a free kinesin head to microtubule. Additional aspects of kinesin's motion, such as head coordination and rate-limiting steps, are directly related to the force-dependent inhibition of ATP binding to a microtubule bound head. The model of rectified Brownian motion is presented as an alternative to power stroke models and provides an alternative interpretation for the significance of ATP hydrolysis in the kinesin stepping cycle.
Figures


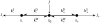
Similar articles
-
Kinesin's walk: springy or gated head coordination?Biosystems. 2009 May;96(2):121-6. doi: 10.1016/j.biosystems.2008.12.002. Epub 2008 Dec 27. Biosystems. 2009. PMID: 19150481
-
Kinesin's second step.Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3444-9. doi: 10.1073/pnas.0307691101. Epub 2004 Feb 25. Proc Natl Acad Sci U S A. 2004. PMID: 14985504 Free PMC article.
-
Searching for kinesin's mechanical amplifier.Philos Trans R Soc Lond B Biol Sci. 2000 Apr 29;355(1396):449-57. doi: 10.1098/rstb.2000.0586. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10836498 Free PMC article. Review.
-
Kinesin: a molecular motor with a spring in its step.Proc Biol Sci. 2002 Nov 22;269(1507):2363-71. doi: 10.1098/rspb.2002.2117. Proc Biol Sci. 2002. PMID: 12495505 Free PMC article. Review.
-
A chemically reversible Brownian motor: application to kinesin and Ncd.Biophys J. 1999 Aug;77(2):993-1002. doi: 10.1016/S0006-3495(99)76950-X. Biophys J. 1999. PMID: 10423444 Free PMC article.
Cited by
-
Kinesin-5: cross-bridging mechanism to targeted clinical therapy.Gene. 2013 Dec 1;531(2):133-49. doi: 10.1016/j.gene.2013.08.004. Epub 2013 Aug 14. Gene. 2013. PMID: 23954229 Free PMC article. Review.
-
Towards a cure for dementia: the role of axonal transport in Alzheimer's disease.Sci Prog. 2008;91(Pt 1):65-80. doi: 10.3184/003685008X285375. Sci Prog. 2008. PMID: 18453283 Free PMC article. Review.
-
Kinesins with extended neck linkers: a chemomechanical model for variable-length stepping.Bull Math Biol. 2012 May;74(5):1066-97. doi: 10.1007/s11538-011-9697-6. Epub 2011 Oct 14. Bull Math Biol. 2012. PMID: 21997362 Free PMC article.
-
Neck-linker docking coordinates the kinetics of kinesin's heads.Biophys J. 2011 Apr 6;100(7):1729-36. doi: 10.1016/j.bpj.2011.01.039. Biophys J. 2011. PMID: 21463586 Free PMC article.
-
An ATP gate controls tubulin binding by the tethered head of kinesin-1.Science. 2007 Apr 6;316(5821):120-3. doi: 10.1126/science.1136985. Science. 2007. PMID: 17412962 Free PMC article.
References
-
- Carter, N. J., and R. A. Cross. 2005. Mechanics of the kinesin step. Nature. 435:308–312. - PubMed
-
- Fox, R. F. 1998. Rectified Brownian movement in molecular and cell biology. Phys. Rev. E. 57:2177–2203.
-
- Rice, S., A. W. Lin, D. Safer, C. L. Hart, N. Naber, B. O. Carragher, S. M. Cain, E. Pechatnikova, E. M. Wilson-Kubalek, M. Whittaker, E. Pate, R. Cooke, E. W. Taylor, R. A. Milligan, and R. D. Vale. 1999. A structural change in the kinesin motor protein that drives motility. Nature. 402:778–784. - PubMed
-
- Fox, R. F., and M. H. Choi. 2001. Rectified Brownian motion and kinesin motion along microtubules. Phys. Rev. E. 63:051901. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

