A Brownian dynamics study of the interactions of the luminal domains of the cytochrome b6f complex with plastocyanin and cytochrome c6: the effects of the Rieske FeS protein on the interactions
- PMID: 16844750
- PMCID: PMC1562394
- DOI: 10.1529/biophysj.106.085936
A Brownian dynamics study of the interactions of the luminal domains of the cytochrome b6f complex with plastocyanin and cytochrome c6: the effects of the Rieske FeS protein on the interactions
Abstract
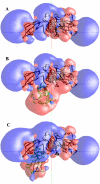
The availability of the structures of the cytochrome b6f complex (cyt b6f), plastocyanin (PC), and cytochrome c6 (cyt c6) from Chlamydomonas reinhardtii allowed us, for the first time, to model electron transfer interactions between the luminal domains of this complex (including cyt f and the Rieske FeS protein) and its redox partners in the same species. We also generated a model structure in which the FeS center of the Rieske protein was positioned closer to the heme of cyt f than observed in the crystal structure and studied its interactions with both PC and cyt c6. Our data showed that the Rieske protein in both the original crystal structure and in our modeled structure of the cyt b6f complex did not physically interfere with binding position or orientation of PC or cyt c6 on cyt f. PC docked on cyt f with the same orientation in the presence or the absence of the Rieske protein, which matched well with the previously reported NMR structures of complexes between cyt f and PC. When the FeS center of the Rieske protein was moved close to the heme of cyt f, it even enhanced the interaction rates. Studies using a cyt f modified in the 184-191 loop showed that the cyt f structure is a more important factor in determining the rate of complex formations than is the presence or the absence of the Rieske protein or its position with respect to cyt f.
Figures





Similar articles
-
Brownian dynamics study of cytochrome f interactions with cytochrome c6 and plastocyanin in Chlamydomonas reinhardtii plastocyanin, and cytochrome c6 mutants.Biophys J. 2005 Mar;88(3):2323-39. doi: 10.1529/biophysj.104.053561. Epub 2004 Dec 30. Biophys J. 2005. PMID: 15626695 Free PMC article.
-
Brownian dynamics simulations of the interaction of Chlamydomonas cytochrome f with plastocyanin and cytochrome c6.Biophys J. 2003 Sep;85(3):2055-68. doi: 10.1016/S0006-3495(03)74633-5. Biophys J. 2003. PMID: 12944318 Free PMC article.
-
A Brownian dynamics study of the effects of cytochrome f structure and deletion of its small domain in interactions with cytochrome c6 and plastocyanin in Chlamydomonas reinhardtii.Biophys J. 2006 Jan 15;90(2):566-77. doi: 10.1529/biophysj.105.067058. Epub 2005 Oct 20. Biophys J. 2006. PMID: 16239335 Free PMC article.
-
[Structural studies on the photosynthetic electron transfer complexes].Tanpakushitsu Kakusan Koso. 2005 Aug;50(10 Suppl):1167-73. Tanpakushitsu Kakusan Koso. 2005. PMID: 16104581 Review. Japanese. No abstract available.
-
Close encounters of the transient kind: protein interactions in the photosynthetic redox chain investigated by NMR spectroscopy.Acc Chem Res. 2003 Oct;36(10):723-30. doi: 10.1021/ar0200955. Acc Chem Res. 2003. PMID: 14567705 Review.
Cited by
-
Fundamental aspects of protein-protein association kinetics.Chem Rev. 2009 Mar 11;109(3):839-60. doi: 10.1021/cr800373w. Chem Rev. 2009. PMID: 19196002 Free PMC article. Review. No abstract available.
-
Using Coarse-Grained Simulations to Characterize the Mechanisms of Protein-Protein Association.Biomolecules. 2020 Jul 15;10(7):1056. doi: 10.3390/biom10071056. Biomolecules. 2020. PMID: 32679892 Free PMC article.
-
Integrating Structural Information to Study the Dynamics of Protein-Protein Interactions in Cells.Structure. 2018 Oct 2;26(10):1414-1424.e3. doi: 10.1016/j.str.2018.07.010. Epub 2018 Aug 30. Structure. 2018. PMID: 30174150 Free PMC article.
-
Predicting Protein-protein Association Rates using Coarse-grained Simulation and Machine Learning.Sci Rep. 2017 Apr 18;7:46622. doi: 10.1038/srep46622. Sci Rep. 2017. PMID: 28418043 Free PMC article.
-
Electrostatic rate enhancement and transient complex of protein-protein association.Proteins. 2008 Apr;71(1):320-35. doi: 10.1002/prot.21679. Proteins. 2008. PMID: 17932929 Free PMC article.
References
-
- Kurisu, G., H. Zhang, J. L. Smith, and W. A. Cramer. 2003. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science. 302:1009–1014. - PubMed
-
- Stroebel, D., Y. Choquet, J. L. Popot, and D. Picot. 2003. An atypical heme in the cytochrome b6f complex. Nature. 426:413–418. - PubMed
-
- Smith, J. L., H. Zhang, J. Yan, G. Kurisu, and W. A. Cramer. 2004. Cytochrome bc complexes: a common core of structure and function surrounded by diversity in the outlying provinces. Curr. Opin. Struct. Biol. 14:432–439. - PubMed
-
- Hope, A. B. 2000. Electron transfers amongst cytochrome f, plastocyanin and photosystem I: kinetics and mechanisms. Biochim. Biophys. Acta. 1456:5–26. - PubMed
-
- Xia, D., C.-A. Yu, H. Kim, J.-Z. Xia, A. M. Kachurin, L. Zhang, L. Yu, and J. Deisenhofer. 1997. Crystal structures of the cytochrome bc1 complex from bovine heart mitochondria. Science. 227:60–66. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

