Benefit of farnesoid X receptor inhibition in obstructive cholestasis
- PMID: 16844773
- PMCID: PMC1544085
- DOI: 10.1073/pnas.0604772103
Benefit of farnesoid X receptor inhibition in obstructive cholestasis
Abstract
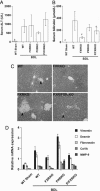
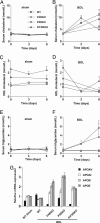
The nuclear hormone receptors farnesoid X receptor (FXR) and pregnane X receptor have been implicated in regulating bile acid, lipid, carbohydrate, and xenobiotic metabolism. Bile duct ligation was used to increase endogenous bile acids and evaluate the roles of these receptors in modulating cholestatic liver injury. FXR knockout (KO) mice were found to be protected from obstructive cholestasis. Concurrent deletion of FXR also could ameliorate an increase in liver injury that is seen usually in pregnane X receptor KO mice with cholestasis. Mechanisms proposed for this protection include the lowering of bile acid concentrations and altered expression of the hepatic transporters Mdr1, Mdr2, BSEP, and Mrp4. FXR KO mice also exhibit a biphasic lipid profile after bile duct ligation, with an increase in high-density lipoprotein cholesterol and triglycerides by day 6. The expression of apolipoprotein AV was reduced in these mice, implicating FXR in triglyceride regulation. We show that FXR modulates cholestasis by controlling bile acids within the hepatocyte and is involved in bile acid synthesis, bile excretion via BSEP, and serum export via Mrp4. This study strongly suggests a potential clinical role for FXR antagonists in the treatment of obstructive cholestatic liver disorders.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Russell D. W. Annu. Rev. Biochem. 2003;72:137–174. - PubMed
-
- Sirvent A., Claudel T., Martin G., Brozek J., Kosykh V., Darteil R., Hum D. W., Fruchart J. C., Staels B. FEBS Lett. 2004;566:173–177. - PubMed
-
- Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. Cell. 2000;102:731–744. - PubMed
-
- Staudinger J. L., Madan A., Carol K. M., Parkinson A. Drug Metab. Dispos. 2003;31:523–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

