Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration
- PMID: 16844785
- PMCID: PMC1544079
- DOI: 10.1073/pnas.0602131103
Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration
Abstract
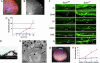
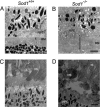
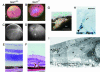
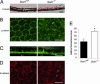
Oxidative stress has long been linked to the pathogenesis of neurodegenerative diseases; however, whether it is a cause or merely a consequence of the degenerative process is still unknown. We show that mice deficient in Cu, Zn-superoxide dismutase (SOD1) have features typical of age-related macular degeneration in humans. Investigations of senescent Sod1(-/-) mice of different ages showed that the older animals had drusen, thickened Bruch's membrane, and choroidal neovascularization. The number of drusen increased with age, and exposure of young Sod1(-/-) mice to excess light induced drusen. The retinal pigment epithelial cells of Sod1(-/-) mice showed oxidative damage, and their beta-catenin-mediated cellular integrity was disrupted, suggesting that oxidative stress may affect the junctional proteins necessary for the barrier integrity of the retinal pigment epithelium. These observations strongly suggest that oxidative stress may play a causative role in age-related retinal degeneration, and our findings provide evidence for the free radical theory of aging. In addition, these results demonstrate that the Sod1(-/-) mouse is a valuable animal model to study human age-related macular degeneration.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Sommer A., Tielsch J. M., Katz J., Quigley H. A., Gottsch J. D., Javitt J. C., Martone J. F., Royall R. M., Witt K. A., Ezrine S. N. Engl. J. Med. 1991;325:1412–1417. - PubMed
-
- Smith W., Assink J., Klein R., Mitchell P., Klaver C. C., Klein B. E., Hofman A., Jensen S., Wang J. J., de Jong P. T. Ophthalmology. 2001;108:697–704. - PubMed
-
- Attbo K., Mitchell P., Smith W. Ophthalmology. 1996;103:357–364. - PubMed
-
- VanNewkirk M. R., Weih L., McCarty C. A., Taylor H. R. Ophthalmology. 2001;108:960–967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

