Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves
- PMID: 16844834
- PMCID: PMC1557605
- DOI: 10.1104/pp.106.082115
Wounding stimulates the accumulation of glycerolipids containing oxophytodienoic acid and dinor-oxophytodienoic acid in Arabidopsis leaves
Abstract
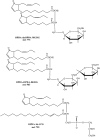
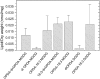
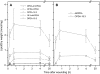
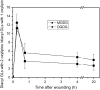
Although oxylipins can be synthesized from free fatty acids, recent evidence suggests that oxylipins are components of plastid-localized polar complex lipids in Arabidopsis (Arabidopsis thaliana). Using a combination of electrospray ionization (ESI) collisionally induced dissociation time-of-flight mass spectrometry (MS) to identify acyl chains, ESI triple-quadrupole (Q) MS in the precursor mode to identify the nominal masses of complex polar lipids containing each acyl chain, and ESI Q-time-of-flight MS to confirm the identifications of the complex polar lipid species, 17 species of oxylipin-containing phosphatidylglycerols, monogalactosyldiacylglycerols (MGDG), and digalactosyldiacylglycerols (DGDG) were identified. The oxylipins of these polar complex lipid species include oxophytodienoic acid (OPDA), dinor-OPDA (dnOPDA), 18-carbon ketol acids, and 16-carbon ketol acids. Using ESI triple-Q MS in the precursor mode, the accumulation of five OPDA- and/or dnOPDA-containing MGDG and two OPDA-containing DGDG species were monitored as a function of time in mechanically wounded leaves. In unwounded leaves, the levels of these oxylipin-containing complex lipid species were low, between 0.001 and 0.023 nmol/mg dry weight. However, within the first 15 min after wounding, the levels of OPDA-dnOPDA MGDG, OPDA-OPDA MGDG, and OPDA-OPDA DGDG, each containing two oxylipin chains, increased 200- to 1,000-fold. In contrast, levels of OPDA-hexadecatrienoic acid MGDG, linolenic acid (18:3)-dnOPDA MGDG, OPDA-18:3 MGDG, and OPDA-18:3 DGDG, each containing a single oxylipin chain, rose 2- to 9-fold. The rapid accumulation of high levels of galactolipid species containing OPDA-OPDA and OPDA-dnOPDA in wounded leaves is consistent with these lipids being the primary products of plastidic oxylipin biosynthesis.
Figures




Similar articles
-
Acylated monogalactosyl diacylglycerol: prevalence in the plant kingdom and identification of an enzyme catalyzing galactolipid head group acylation in Arabidopsis thaliana.Plant J. 2015 Dec;84(6):1152-66. doi: 10.1111/tpj.13072. Plant J. 2015. PMID: 26566971
-
High-resolution profiling of oxylipin-containing galactolipids in Arabidopsis extracts by ultra-performance liquid chromatography/time-of-flight mass spectrometry.Rapid Commun Mass Spectrom. 2008 Oct;22(20):3154-60. doi: 10.1002/rcm.3716. Rapid Commun Mass Spectrom. 2008. PMID: 18798198
-
A predicted plastid rhomboid protease affects phosphatidic acid metabolism in Arabidopsis thaliana.Plant J. 2019 Sep;99(5):978-987. doi: 10.1111/tpj.14377. Epub 2019 Jun 7. Plant J. 2019. PMID: 31062431 Free PMC article.
-
The function of the oxylipin 12-oxophytodienoic acid in cell signaling, stress acclimation, and development.J Exp Bot. 2018 Nov 26;69(22):5341-5354. doi: 10.1093/jxb/ery316. J Exp Bot. 2018. PMID: 30169821 Review.
-
Head-Group Acylation of Chloroplast Membrane Lipids.Molecules. 2021 Feb 26;26(5):1273. doi: 10.3390/molecules26051273. Molecules. 2021. PMID: 33652855 Free PMC article. Review.
Cited by
-
Fatty Acid Composition by Total Acyl Lipid Collision-Induced Dissociation Time-of-Flight (TAL-CID-TOF) Mass Spectrometry.Methods Mol Biol. 2021;2295:117-133. doi: 10.1007/978-1-0716-1362-7_8. Methods Mol Biol. 2021. PMID: 34047975 Free PMC article.
-
HILIC-ESI-MS analysis of phosphatidic acid methyl esters artificially generated during lipid extraction from microgreen crops.J Mass Spectrom. 2021 Aug 28;56(10):e4784. doi: 10.1002/jms.4784. Online ahead of print. J Mass Spectrom. 2021. PMID: 34528340 Free PMC article.
-
Mass spectrometry imaging of Arabidopsis thaliana with in vivo D2O labeling.Front Plant Sci. 2024 May 31;15:1379299. doi: 10.3389/fpls.2024.1379299. eCollection 2024. Front Plant Sci. 2024. PMID: 38882571 Free PMC article.
-
The AtMYB60 transcription factor regulates stomatal opening by modulating oxylipin synthesis in guard cells.Sci Rep. 2022 Jan 11;12(1):533. doi: 10.1038/s41598-021-04433-y. Sci Rep. 2022. PMID: 35017563 Free PMC article.
-
Stepwise Biogenesis of Subpopulations of Lipid Droplets in Nitrogen Starved Phaeodactylum tricornutum Cells.Front Plant Sci. 2020 Feb 11;11:48. doi: 10.3389/fpls.2020.00048. eCollection 2020. Front Plant Sci. 2020. PMID: 32117386 Free PMC article.
References
-
- Bachmann A, Hause B, Maucher H, Garbe E, Voros K, Weichert H, Wasternack C, Feussner I (2002) Jasmonate-induced lipid peroxidation in barley leaves initiated by distinct 13-LOX forms of chloroplasts. J Biol Chem 383: 1645–1657 - PubMed
-
- Blée E (1998) Phytooxylipins and plant defense reactions. Prog Lipid Res 37: 33–72 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

