Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene
- PMID: 16845369
- PMCID: PMC1559663
- DOI: 10.1038/sj.embor.7400757
Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene
Abstract
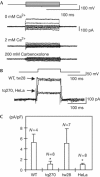
Leopard, a well-known zebrafish mutant that has a spotted skin pattern instead of stripes, is a model for the study of pigment patterning. To understand the mechanisms underlying stripe formation, as well as the spot variation observed in leopard, we sought to identify the gene responsible for this phenotype. Using positional cloning, we identified the leopard gene as an orthologue of the mammalian connexin 40 gene. A variety of different leopard alleles, such as leo(t1), leo(tq270) and leo(tw28), show different skin-pattern phenotypes. In this manuscript we show that the mutation in allele leo(t1) is a nonsense mutation, whereas alleles leo(tq270) and leo(tw28) contain the missense mutations I202F and I31F, respectively. Patch-clamp experiments of connexin hemichannels demonstrated that the I202F substitution in allele leo(tq270) disrupted the channel function of connexin41.8. These results demonstrate that mutations in this gene lead to a variety of leopard spot patterns.
Figures


Similar articles
-
Gap junctions composed of connexins 41.8 and 39.4 are essential for colour pattern formation in zebrafish.Elife. 2014 Dec 23;3:e05125. doi: 10.7554/eLife.05125. Elife. 2014. PMID: 25535837 Free PMC article.
-
The Physiological Characterization of Connexin41.8 and Connexin39.4, Which Are Involved in the Striped Pattern Formation of Zebrafish.J Biol Chem. 2016 Jan 15;291(3):1053-63. doi: 10.1074/jbc.M115.673129. Epub 2015 Nov 23. J Biol Chem. 2016. PMID: 26598520 Free PMC article.
-
Changing clothes easily: connexin41.8 regulates skin pattern variation.Pigment Cell Melanoma Res. 2012 May;25(3):326-30. doi: 10.1111/j.1755-148X.2012.00984.x. Epub 2012 Feb 29. Pigment Cell Melanoma Res. 2012. PMID: 22313791
-
Evolution of danio pigment pattern development.Heredity (Edinb). 2006 Sep;97(3):200-10. doi: 10.1038/sj.hdy.6800867. Epub 2006 Jul 12. Heredity (Edinb). 2006. PMID: 16835593 Review.
-
The Developmental Genetics of Vertebrate Color Pattern Formation: Lessons from Zebrafish.Curr Top Dev Biol. 2016;117:141-69. doi: 10.1016/bs.ctdb.2015.12.012. Epub 2016 Feb 23. Curr Top Dev Biol. 2016. PMID: 26969976 Review.
Cited by
-
Melanophore migration and survival during zebrafish adult pigment stripe development require the immunoglobulin superfamily adhesion molecule Igsf11.PLoS Genet. 2012;8(8):e1002899. doi: 10.1371/journal.pgen.1002899. Epub 2012 Aug 16. PLoS Genet. 2012. PMID: 22916035 Free PMC article.
-
Fishing forward and reverse: Advances in zebrafish phenomics.Mech Dev. 2018 Dec;154:296-308. doi: 10.1016/j.mod.2018.08.007. Epub 2018 Aug 18. Mech Dev. 2018. PMID: 30130581 Free PMC article. Review.
-
Neural crest cells as a source of microevolutionary variation.Semin Cell Dev Biol. 2023 Aug;145:42-51. doi: 10.1016/j.semcdb.2022.06.001. Epub 2022 Jun 16. Semin Cell Dev Biol. 2023. PMID: 35718684 Free PMC article. Review.
-
Origins of adult pigmentation: diversity in pigment stem cell lineages and implications for pattern evolution.Pigment Cell Melanoma Res. 2015 Jan;28(1):31-50. doi: 10.1111/pcmr.12332. Epub 2014 Dec 16. Pigment Cell Melanoma Res. 2015. PMID: 25421288 Free PMC article. Review.
-
A connexin/ifi30 pathway bridges HSCs with their niche to dampen oxidative stress.Nat Commun. 2021 Jul 23;12(1):4484. doi: 10.1038/s41467-021-24831-0. Nat Commun. 2021. PMID: 34301940 Free PMC article.
References
-
- Asai R, Taguchi E, Kume Y, Saito M, Kondo S (1999) Zebrafish leopard gene as a component of the putative reaction–diffusion system. Mech Dev 89: 87–92 - PubMed
-
- Bao X, Reuss L, Altenberg GA (2004) Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J Biol Chem 279: 20058–20066 - PubMed
-
- Bevans CG, Kordel M, Rhee SK, Harris AL (1998) Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem 273: 2808–2816 - PubMed
-
- Christie TL, Mui R, White TW, Valdimarsson G (2004) Molecular cloning, functional analysis, and RNA expression analysis of connexin45.6: a zebrafish cardiovascular connexin. Am J Physiol Heart Circ Physiol 286: H1623–H1632 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

