Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis
- PMID: 16845371
- PMCID: PMC1525155
- DOI: 10.1038/sj.embor.7400752
Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis
Abstract
Growth differentiation factor 11 (GDF11) contributes to regionalize the mouse embryo along its anterior-posterior axis by regulating the expression of Hox genes. The identity of the receptors that mediate GDF11 signalling during embryogenesis remains unclear. Here, we show that GDF11 can interact with type I receptors ALK4, ALK5 and ALK7, but predominantly uses ALK4 and ALK5 to activate a Smad3-dependent reporter gene. Alk5 mutant embryos showed malformations in anterior-posterior patterning, including the lack of expression of the posterior determinant Hoxc10, that resemble defects found in Gdf11-null mutants. A heterozygous mutation in Alk5, but not in Alk4 or Alk7, potentiated Gdf11(-/-)-like phenotypes in vertebral, kidney and palate development in an Acvr2b(-/-) background, indicating a genetic interaction between the two receptor genes. Thus, the transforming growth factor-beta (TGF-beta) receptor ALK5, which until now has only been associated with the biological functions of TGF-beta1 to TGF-beta3 proteins, mediates GDF11 signalling during embryogenesis.
Figures
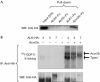
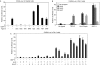

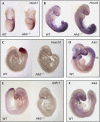

References
-
- Andersson O, Reissmann E, Jörnvall H, Ibáñez CF (2006) Synergistic interaction between Gdf1 and Nodal during anterior axis development. Dev Biol 293: 370–381 - PubMed
-
- Chang H, Brown CW, Matzuk MM (2002) Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev 23: 787–823 - PubMed
-
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW (2006) The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development 133: 319–329 - PubMed
-
- Deschamps J, van Nes J (2005) Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development 132: 2931–2942 - PubMed
-
- Dubrulle J, Pourquie O (2004) Coupling segmentation to axis formation. Development 131: 5783–5793 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

