Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force
- PMID: 16845394
- PMCID: PMC1764822
- DOI: 10.1038/ni1366
Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force
Abstract
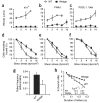
Crystal structures of the lectin and epidermal growth factor (EGF)-like domains of P-selectin show 'bent' and 'extended' conformations. An extended conformation would be 'favored' by forces exerted on a selectin bound at one end to a ligand and at the other end to a cell experiencing hydrodynamic drag forces. To determine whether the extended conformation has higher affinity for ligand, we introduced an N-glycosylation site to 'wedge open' the interface between the lectin and EGF-like domains of P-selectin. This alteration increased the affinity of P-selectin for its ligand P-selectin glycoprotein 1 (PSGL-1) and thereby the strength of P-selectin-mediated rolling adhesion. Similarly, an asparagine-to-glycine substitution in the lectin-EGF-like domain interface of L-selectin enhanced rolling adhesion under shear flow. Our results demonstrate that force, by 'favoring' an extended selectin conformation, can strengthen selectin-ligand bonds.
Figures




References
-
- Rosen SD, Bertozzi CR. The selectins and their ligands. Curr. Opin. Cell Biol. 1994;6:663–673. - PubMed
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multi-step paradigm. Cell. 1994;76:301–314. - PubMed
-
- Kansas GS. Selectins and their ligands: Current concepts and controversies. Blood. 1996;88:3259–3287. - PubMed
-
- Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999;79:181–213. - PubMed
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

