Antiandrogen treatments in locally advanced prostate cancer: are they all the same?
- PMID: 16845534
- PMCID: PMC12161114
- DOI: 10.1007/s00432-006-0133-5
Antiandrogen treatments in locally advanced prostate cancer: are they all the same?
Abstract
Purpose: The objectives are to review the published literature and to evaluate the weight of evidence for clinical effectiveness, safety, and tolerability of the currently available antiandrogens in the treatment of locally advanced prostate cancer. This article covers efficacy as monotherapy relative to castration and as adjuvant to radiotherapy and radical prostatectomy as well as adverse-effect and quality-of-life data.
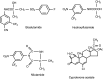
Methods: The current literature from online databases between 1986 and the present, relating to antiandrogen treatments in men with locally advanced disease given either as monotherapy or as adjuvant to radical radiotherapy or prostatectomy, was reviewed. Antiandrogens researched included the non-steroidal antiandrogens, bicalutamide ('Casodex'), flutamide, and nilutamide, and the steroidal antiandrogen cyproterone acetate (CPA).
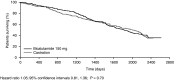
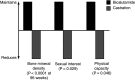
Results: The most comprehensively investigated and reported antiandrogen is bicalutamide, which has shown survival outcomes similar to those observed with castration in patients with locally advanced prostate cancer. In contrast, only limited clinical data are available for the other non-steroidal antiandrogens (flutamide and nilutamide) and the steroidal antiandrogen CPA in patients with locally advanced disease. In terms of safety and tolerability, CPA is associated with loss of libido and erectile dysfunction. CPA is also associated with cardiovascular risk and there have been occasional reports of fatal fulminant hepatitis and hepatocellular carcinoma. Gynecomastia is quite rare with CPA, which is in contrast to the non-steroidal antiandrogens. There are no direct comparisons between the three non-steroidal antiandrogens in terms of quality of life, but available evidence suggests that bicalutamide has a more favorable safety and tolerability profile than nilutamide and flutamide. Unlike CPA, non-steroidal antiandrogens appear to be better tolerated than castration, allowing patients to maintain sexual activity, physical ability, and bone mineral density, but these agents have a higher incidence of gynecomastia and breast pain (mild to moderate in > 90% of cases). Gynecomastia and breast pain, however, can be effectively managed.
Conclusions: The available evidence indicates that the different antiandrogens should not be regarded as equivalents in clinical practice and so the choice of treatment for patients with prostate cancer should be made on an individual basis. It is, therefore, important for clinicians to discuss the efficacy and tolerability profiles of all available treatment options with their patients to enable them to choose a treatment program that best fits with their lifestyle.
Figures
Similar articles
-
What implications do the tolerability profiles of antiandrogens and other commonly used prostate cancer treatments have on patient care?J Cancer Res Clin Oncol. 2006 Aug;132 Suppl 1(Suppl 1):S27-35. doi: 10.1007/s00432-006-0134-4. J Cancer Res Clin Oncol. 2006. PMID: 16896883 Free PMC article. Review.
-
Pharmacological interventions for those who have sexually offended or are at risk of offending.Cochrane Database Syst Rev. 2015 Feb 18;2015(2):CD007989. doi: 10.1002/14651858.CD007989.pub2. Cochrane Database Syst Rev. 2015. PMID: 25692326 Free PMC article.
-
Interventions for hirsutism (excluding laser and photoepilation therapy alone).Cochrane Database Syst Rev. 2015 Apr 28;2015(4):CD010334. doi: 10.1002/14651858.CD010334.pub2. Cochrane Database Syst Rev. 2015. PMID: 25918921 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
New insights into the androgen-targeted therapies and epigenetic therapies in prostate cancer.Prostate Cancer. 2011;2011:918707. doi: 10.1155/2011/918707. Epub 2011 Oct 12. Prostate Cancer. 2011. PMID: 22111003 Free PMC article.
-
Exploring anti-androgen therapies in hormone dependent prostate cancer and new therapeutic routes for castration resistant prostate cancer.Front Endocrinol (Lausanne). 2022 Oct 3;13:1006101. doi: 10.3389/fendo.2022.1006101. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36263323 Free PMC article. Review.
-
Coactivator selective regulation of androgen receptor activity.Steroids. 2009 Aug;74(8):669-74. doi: 10.1016/j.steroids.2009.02.007. Epub 2009 Mar 9. Steroids. 2009. PMID: 19463689 Free PMC article.
-
Novel insights into post-marketing AEs associated with leuprorelin: A comprehensive analysis utilizing the FAERS database.Heliyon. 2024 Jul 21;10(15):e34969. doi: 10.1016/j.heliyon.2024.e34969. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39157412 Free PMC article.
-
Second brazilian consensus on the treatment of advanced prostate cancer - a SBOC-SBU-SBRT panel review.Int Braz J Urol. 2019 May-Jun;45(3):449-458. doi: 10.1590/S1677-5538.IBJU.2018.0798. Int Braz J Urol. 2019. PMID: 31038861 Free PMC article.
References
-
- Adelson KB, Loprinzi CL, Hershman DL (2005) Treatment of hot flushes in breast and prostate cancer. Expert Opin Pharmacother 6:1095–1106 - PubMed
-
- Anderson J (2003) The role of antiandrogen monotherapy in the treatment of prostate cancer. BJU Int 91:455–461 - PubMed
-
- Aus G, Abbou CC, Bolla M, Heidenreich A, Van Poppel H, Schmid H-P, Wolff JM, Zattoni F (2005) European Association of Urology guidelines on prostate cancer. http://www.uroweb.nl/files/uploaded_files/2005Prostate%20Cancer.pdf (accessed 19 December 2005) - PubMed
-
- Barradell LB, Faulds D (1994) Cyproterone. A review of its pharmacology and therapeutic efficacy in prostate cancer. Drugs Aging 5:59–80 - PubMed
-
- Berruti A, Dogliotti L, Terrone C, Cerruti S, Isaia G, Tarabuzzi R, Reimondo G, Mari M, Ardissone P, De Luca S, Fasolis G, Fontana D, Rossetti SR, Angeli A, Gruppo Onco Urologico Piemontese (GOUP) ROP (2002) Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation. J Urol 167:2361–2367 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

