Spectroscopic analysis of benzylidene anabaseine complexes with acetylcholine binding proteins as models for ligand-nicotinic receptor interactions
- PMID: 16846232
- PMCID: PMC3222595
- DOI: 10.1021/bi060534y
Spectroscopic analysis of benzylidene anabaseine complexes with acetylcholine binding proteins as models for ligand-nicotinic receptor interactions
Abstract
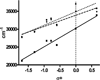
The discovery of the acetylcholine binding proteins (AChBPs) has provided critical soluble surrogates for examining structure and ligand interactions with nicotinic receptors and related pentameric ligand-gated ion channels. The multiple marine and freshwater sources of AChBP constitute a protein family with substantial sequence divergence and selectivity in ligand recognition for analyzing structure-activity relationships. The purification of AChBP in substantial quantities in the absence of a detergent enables one to conduct spectroscopic studies of the ligand-AChBP complexes. To this end, we have examined the interaction of a congeneric series of benzylidene-ring substituted anabaseines with AChBPs from Lymnaea, Aplysia, and Bulinus species and correlated their binding energetics with spectroscopic changes associated with ligand binding. The anabaseines display agonist activity on the alpha7 nicotinic receptor, a homomeric receptor with sequences similar to those of the AChBPs. Substituted anabaseines show absorbance and fluorescence properties sensitive to the protonation state, relative permittivity (dielectric constant), and the polarizability of the surrounding solvent or the proximal residues in the binding site. Absorbance difference spectra reveal that a single protonation state of the ligand binds to AChBP and that the bound ligand experiences a solvent environment with a high degree of polarizability. Changes in the fluorescence quantum yield of the bound ligand reflect the rigidification of the ring system of the bound ligand. Hence, the spectral properties of the bound ligand allow a description of the electronic character of the bound state of the ligand within its aromatic binding pocket and provide information complementary to that of crystal structures in defining the determinants of interaction.
Figures






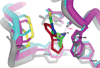

References
-
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. - PubMed
-
- Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, Lodder H, van der Schors RC, van Elk R, Sorgedrager B, Brejc K, Sixma TK, Geraerts WP. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;411:261–268. - PubMed
-
- Celie PH, Klaassen RV, van Rossum-Fikkert SE, van Elk R, van Nierop P, Smit AB, Sixma TK. Crystal structure of acetylcholine-binding protein from Bulinus truncatus reveals the conserved structural scaffold and sites of variation in nicotinic acetylcholine receptors. J. Biol. Chem. 2005;280:26457–26466. - PubMed
-
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41:907–914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

