Shigella sonnei biotype G carrying class 2 integrons in southern Italy: a retrospective typing study by pulsed field gel electrophoresis
- PMID: 16846516
- PMCID: PMC1538607
- DOI: 10.1186/1471-2334-6-117
Shigella sonnei biotype G carrying class 2 integrons in southern Italy: a retrospective typing study by pulsed field gel electrophoresis
Abstract
Background: Emergence and global dissemination of multiresistant strains of enteric pathogens is a very concerning problem from both epidemiological and Public Health points of view. Shigella sonnei is the serogroup of Shigella most frequently responsible for sporadic and epidemic enteritis in developed countries. The dissemination is associated most often to human to human transmission, but foodborne episodes have also been described. In recent years the circulation of multiresistant strains of S. sonnei biotype g carrying a class 2 integron has been reported in many countries worldwide. In southern Italy a strain with similar properties has been responsible for a large community outbreak occurred in 2003 in Palermo, Sicily. The objective of this study was to date the emergence of the biotype g strain carrying the class 2 integron in southern Italy and to evaluate the genetic heterogeneity of biotype g S. sonnei isolated throughout an extended interval of time.
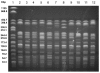
Methods: A total of 31 clinical isolates of S. sonnei biotype g identified in southern Italy during the years 1971-2000 were studied. The strains were identified at the serogroup level, characterized by biochemical tests and submitted to antimicrobial susceptibility testing. Molecular typing was performed by pulsed field gel electrophoresis (PFGE) after digestion of DNA by XbaI. Carriage of class 2 integrons was investigated by polymerase chain reaction (PCR) with specific primers and confirmed by restriction endonuclease analysis of amplicons.
Results: The 15 isolates of S. sonnei biotype g identified in the decade 1971-1980 showed highly heterogeneous drug resistance profiles and pulsotypes. None of the isolates was simultaneous resistant to streptomycin and trimethoprim and none was class 2 integron positive. On the contrary, this resistance phenotype and class 2 integron carriage were very common among the 16 strains of biotype g identified in the following two decades. Moreover, all the more recent isolates, but one, showed closely related pulsotypes.
Conclusion: Although our findings refer to a limited geographic area, they provide a snapshot of integron acquisition by an enteric pathogen responsible for several outbreaks in the years 2001-2003 in Italy. Molecular typing, indeed, suggests that the emergence of biotype g class 2 integron carrying S. sonnei in southern Italy should be backdated to at least the late 1980s. In the following decades, the circulation of biotype g appears to be sustained by multiresistant highly related strains. Similar trend are described in several countries, but the questions about mechanism of emergence and worldwide spread of this pathogen remain open.
Figures

References
-
- Centers for Disease Control and Prevention (CDC) Day care-related outbreaks of rhamnose-negative Shigella sonnei – six states, June 2001-March 2003. MMWR. 2004;53:60–63. - PubMed
-
- DeLappe N, O'Halloran F, Fanning S, Corbett-Feeney G, Cheasty T, Cormican M. Antimicrobial resistance and genetic diversity of Shigella sonnei isolates from western Ireland, an area of low incidence of infection. J Clin Microbiol. 2003;41:1919–1924. doi: 10.1128/JCM.41.5.1919-1924.2003. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

