Autoantibodies against the replication protein A complex in systemic lupus erythematosus and other autoimmune diseases
- PMID: 16846524
- PMCID: PMC1779422
- DOI: 10.1186/ar2000
Autoantibodies against the replication protein A complex in systemic lupus erythematosus and other autoimmune diseases
Abstract
Replication protein A (RPA), a heterotrimer with subunits of molecular masses 70, 32, and 14 kDa, is a single-stranded-DNA-binding factor involved in DNA replication, repair, and recombination. There have been only three reported cases of anti-RPA in systemic lupus erythematosus (SLE) and Sjögren syndrome (SjS). This study sought to clarify the clinical significance of autoantibodies against RPA. Sera from 1,119 patients enrolled during the period 2000 to 2005 were screened by immunoprecipitation (IP) of 35S-labeled K562 cell extract. Antigen-capture ELISA with anti-RPA32 mAb, immunofluorescent antinuclear antibodies (ANA) and western blot analysis with purified RPA were also performed. Our results show that nine sera immunoprecipitated the RPA70-RPA32-RPA14 complex and all were strongly positive by ELISA (titers 1:62,500 to 1:312,500). No additional sera were positive by ELISA and subsequently confirmed by IP or western blotting. All sera showed fine speckled/homogeneous nuclear staining. Anti-RPA was found in 1.4% (4/276) of SLE and 2.5% (1/40) of SjS sera, but not in rheumatoid arthritis (0/35), systemic sclerosis (0/47), or polymyositis/dermatomyositis (0/43). Eight of nine patients were female and there was no racial predilection. Other positive patients had interstitial lung disease, autoimmune thyroiditis/hepatitis C virus/pernicious anemia, or an unknown diagnosis. Autoantibody specificities found in up to 40% of SLE and other diseases, such as anti-nRNP, anti-Sm, anti-Ro, and anti-La, were unusual in anti-RPA-positive sera. Only one of nine had anti-Ro, and zero of nine had anti-nRNP, anti-Sm, anti-La, or anti-ribosomal P antibodies. In summary, high titers of anti-RPA antibodies were found in nine patients (1.4% of SLE and other diseases). Other autoantibodies found in SLE were rare in this subset, suggesting that patients with anti-RPA may form a unique clinical and immunological subset.
Figures
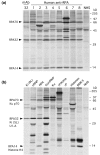

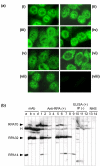
References
-
- Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. - PubMed
-
- Miyachi K, Tan EM. Antibodies reacting with ribosomal ribonucleoprotein in connective tissue diseases. Arthritis Rheum. 1979;22:87–93. - PubMed
-
- Reeves WH, Narain S, Satoh M. Autoantibodies in systemic lupus erythematosus. In: Koopman WJ, Moreland LW, editor. Arthritis and Allied Conditions. 15. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1497–1521.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

