Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer
- PMID: 16846527
- PMCID: PMC1779478
- DOI: 10.1186/bcr1522
Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer
Abstract
Introduction: BRCA1 or BRCA2 germline mutations increase the risk of developing breast cancer. Tumour cells from germline mutation carriers have frequently lost the wild-type allele. This is predicted to result in genomic instability where cell survival depends upon dysfunctional checkpoint mechanisms. Tumorigenic potential could then be acquired through further genomic alterations. Surprisingly, somatic BRCA mutations are not found in sporadic breast tumours. BRCA1 methylation has been shown to occur in sporadic breast tumours and to be associated with reduced gene expression. We examined the frequency of BRCA1 methylation in 143 primary sporadic breast tumours along with BRCA1 copy number alterations and tumour phenotype.
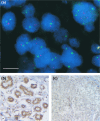
Methods: Primary sporadic breast tumours were analysed for BRCA1alpha promoter methylation by methylation specific PCR and for allelic imbalance (AI) at BRCA1 and BRCA2 loci by microsatellite analysis and TP53 (also known as p53) mutations by constant denaturing gel electrophoresis. The BRCA1 methylated tumours were analysed for BRCA1 copy alterations by fluorescence in situ hybridisation and BRCA1 expression by immunostaining.
Results: BRCA1 methylation was found in 13/143 (9.1%) sporadic breast tumours. The BRCA1 methylated tumours were significantly associated with estrogen receptor (ER) negativity (P = 0.0475) and displayed a trend for BRCA1 AI (P = 0.0731) as well as young-age at diagnosis (< or = 55; P = 0.0898). BRCA1 methylation was not associated with BRCA2 AI (P = 0.5420), although a significant association was found between BRCA1 AI and BRCA2 AI (P < 0.0001).Absent/markedly reduced BRCA1 expression was observed in 9/13 BRCA1 methylated tumours, most of which had BRCA1 deletion. An elevated TP53 mutation frequency was found among BRCA1 methylated tumours (38.5%) compared with non-methylated tumours (17.2%). The BRCA1 methylated tumours were mainly of tumour grade 3 (7/13) and infiltrating ductal type (12/13). Only one methylated tumour was of grade 1.
Conclusion: BRCA1 methylation is frequent in primary sporadic breast tumours. We found an indication for BRCA1 methylation to be associated with AI at the BRCA1 locus. Almost all BRCA1 methylated tumours with absent/markedly reduced BRCA1 expression (8/9) displayed BRCA1 deletion. Thus, epigenetic silencing and deletion of the BRCA1 gene might serve as Knudson's two 'hits' in sporadic breast tumorigenesis. We observed phenotypic similarities between BRCA1 methylated and familial BRCA1 tumours, based on BRCA1 deletion, TP53 mutations, ER status, young age at diagnosis and tumour grade.
Figures


References
-
- Collins N, McManus R, Wooster R, Mangion J, Seal S, Lakhani SR, Ormiston W, Daly PA, Ford D, Easton DF, et al. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12–13. Oncogene. 1995;10:1673–1675. - PubMed
-
- Greenblatt MS, Chappuis PO, Bond JP, Hamel N, Foulkes WD. TP53 mutations in breast cancer associated with BRCA1 or BRCA2 germ-line mutations: distinctive spectrum and structural distribution. Cancer Res. 2001;61:4092–4097. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

