Coexpression and interaction of CXCL10 and CD26 in mesenchymal cells by synergising inflammatory cytokines: CXCL8 and CXCL10 are discriminative markers for autoimmune arthropathies
- PMID: 16846531
- PMCID: PMC1779382
- DOI: 10.1186/ar1997
Coexpression and interaction of CXCL10 and CD26 in mesenchymal cells by synergising inflammatory cytokines: CXCL8 and CXCL10 are discriminative markers for autoimmune arthropathies
Abstract
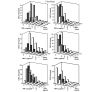
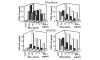
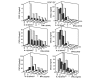
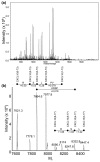
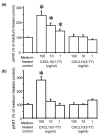
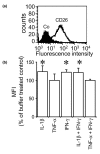
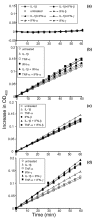
Leukocyte infiltration during acute and chronic inflammation is regulated by exogenous and endogenous factors, including cytokines, chemokines and proteases. Stimulation of fibroblasts and human microvascular endothelial cells with the inflammatory cytokines interleukin-1beta (IL-1beta) or tumour necrosis factor alpha (TNF-alpha) combined with either interferon-alpha (IFN-alpha), IFN-beta or IFN-gamma resulted in a synergistic induction of the CXC chemokine CXCL10, but not of the neutrophil chemoattractant CXCL8. In contrast, simultaneous stimulation with different IFN types did not result in a synergistic CXCL10 protein induction. Purification of natural CXCL10 from the conditioned medium of fibroblasts led to the isolation of CD26/dipeptidyl peptidase IV-processed CXCL10 missing two NH2-terminal residues. In contrast to intact CXCL10, NH2-terminally truncated CXCL10(3-77) did not induce extracellular signal-regulated kinase 1/2 or Akt/protein kinase B phosphorylation in CXC chemokine receptor 3-transfected cells. Together with the expression of CXCL10, the expression of membrane-bound CD26/dipeptidyl peptidase IV was also upregulated in fibroblasts by IFN-gamma, by IFN-gamma plus IL-1beta or by IFN-gamma plus TNF-alpha. This provides a negative feedback for CXCL10-dependent chemotaxis of activated T cells and natural killer cells. Since TNF-alpha and IL-1beta are implicated in arthritis, synovial concentrations of CXCL8 and CXCL10 were compared in patients suffering from crystal arthritis, ankylosing spondylitis, psoriatic arthritis and rheumatoid arthritis. All three groups of autoimmune arthritis patients (ankylosing spondylitis, psoriatic arthritis and rheumatoid arthritis) had significantly increased synovial CXCL10 levels compared with crystal arthritis patients. In contrast, compared with crystal arthritis, only rheumatoid arthritis patients, and not ankylosing spondylitis or psoriatic arthritis patients, had significantly higher synovial CXCL8 concentrations. Synovial concentrations of the neutrophil chemoattractant CXCL8 may therefore be useful to discriminate between autoimmune arthritis types.
Figures


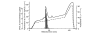

Similar articles
-
Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-gamma and provide a mechanism for enhanced synovial chemokine levels in septic arthritis.Eur J Immunol. 2003 Nov;33(11):3146-53. doi: 10.1002/eji.200324136. Eur J Immunol. 2003. PMID: 14579283
-
Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-gamma in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids.J Leukoc Biol. 2004 May;75(5):777-84. doi: 10.1189/jlb.1003524. Epub 2004 Mar 2. J Leukoc Biol. 2004. PMID: 14996826
-
Cytokines differentially regulate CXCL10 production by interferon-gamma-stimulated or tumor necrosis factor-alpha-stimulated human gingival fibroblasts.J Periodontal Res. 2009 Apr;44(2):225-31. doi: 10.1111/j.1600-0765.2008.01124.x. Epub 2008 Oct 7. J Periodontal Res. 2009. PMID: 18973545
-
Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases.Autoimmun Rev. 2014 Mar;13(3):272-80. doi: 10.1016/j.autrev.2013.10.010. Epub 2013 Nov 2. Autoimmun Rev. 2014. PMID: 24189283 Review.
-
The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection.J Viral Hepat. 2007 Oct;14(10):675-87. doi: 10.1111/j.1365-2893.2006.00838.x. J Viral Hepat. 2007. PMID: 17875002 Review.
Cited by
-
Joint together: The etiology and pathogenesis of ankylosing spondylitis.Front Immunol. 2022 Oct 17;13:996103. doi: 10.3389/fimmu.2022.996103. eCollection 2022. Front Immunol. 2022. PMID: 36325352 Free PMC article. Review.
-
Bioinformatics and system biology approaches to identify the diseasome and comorbidities complexities of SARS-CoV-2 infection with the digestive tract disorders.Brief Bioinform. 2021 Nov 5;22(6):bbab126. doi: 10.1093/bib/bbab126. Brief Bioinform. 2021. PMID: 33993223 Free PMC article.
-
Causal associations between both psoriasis and psoriatic arthritis and multiple autoimmune diseases: a bidirectional two-sample Mendelian randomization study.Front Immunol. 2024 Jul 25;15:1422626. doi: 10.3389/fimmu.2024.1422626. eCollection 2024. Front Immunol. 2024. PMID: 39119335 Free PMC article.
-
On the origin of serum CD26 and its altered concentration in cancer patients.Cancer Immunol Immunother. 2009 Nov;58(11):1723-47. doi: 10.1007/s00262-009-0728-1. Epub 2009 Jun 26. Cancer Immunol Immunother. 2009. PMID: 19557413 Free PMC article. Review.
-
The cGAS-STING signaling pathway in the regulation of pulmonary infections: a systematic review.Front Cell Infect Microbiol. 2025 Jul 8;15:1628481. doi: 10.3389/fcimb.2025.1628481. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40697819 Free PMC article.
References
-
- Van Damme J, Proost P, Put W, Arens S, Lenaerts JP, Conings R, Opdenakker G, Heremans H, Billiau A. Induction of monocyte chemotactic proteins MCP-1 and MCP-2 in human fibroblasts and leukocytes by cytokines and cytokine inducers. Chemical synthesis of MCP-2 and development of a specific RIA. J Immunol. 1994;152:5495–5502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

