Differential expression, function and response to inflammatory stimuli of 11beta-hydroxysteroid dehydrogenase type 1 in human fibroblasts: a mechanism for tissue-specific regulation of inflammation
- PMID: 16846535
- PMCID: PMC1779419
- DOI: 10.1186/ar1993
Differential expression, function and response to inflammatory stimuli of 11beta-hydroxysteroid dehydrogenase type 1 in human fibroblasts: a mechanism for tissue-specific regulation of inflammation
Abstract
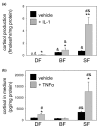
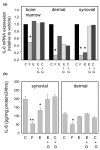
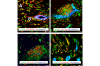
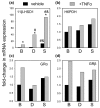
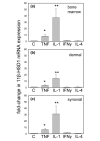
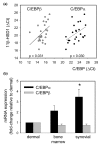
Stromal cells such as fibroblasts play an important role in defining tissue-specific responses during the resolution of inflammation. We hypothesized that this involves tissue-specific regulation of glucocorticoids, mediated via differential regulation of the enzyme 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1). Expression, activity and function of 11beta-HSD1 was assessed in matched fibroblasts derived from various tissues (synovium, bone marrow and skin) obtained from patients with rheumatoid arthritis or osteoarthritis. 11beta-HSD1 was expressed in fibroblasts from all tissues but mRNA levels and enzyme activity were higher in synovial fibroblasts (2-fold and 13-fold higher mRNA levels in dermal and synovial fibroblasts, respectively, relative to bone marrow). Expression and activity of the enzyme increased in all fibroblasts following treatment with tumour necrosis factor-alpha or IL-1beta (bone marrow: 8-fold and 37-fold, respectively, compared to vehicle; dermal fibroblasts: 4-fold and 14-fold; synovial fibroblasts: 7-fold and 31-fold; all P < 0.01 compared with vehicle). Treatment with IL-4 or interferon-gamma was without effect, and there was no difference in 11beta-HSD1 expression between fibroblasts (from any site) obtained from patients with rheumatoid arthritis or osteoarthritis. In the presence of 100 nmol/l cortisone, IL-6 production--a characteristic feature of synovial derived fibroblasts--was significantly reduced in synovial but not dermal or bone marrow fibroblasts. This was prevented by co-treatment with an 11beta-HSD inhibitor, emphasizing the potential for autocrine activation of glucocorticoids in synovial fibroblasts. These data indicate that differences in fibroblast-derived glucocorticoid production (via the enzyme 11beta-HSD1) between cells from distinct anatomical locations may play a key role in the predeliction of certain tissues to develop persistent inflammation.
Figures
Similar articles
-
Local and systemic glucocorticoid metabolism in inflammatory arthritis.Ann Rheum Dis. 2008 Sep;67(9):1204-10. doi: 10.1136/ard.2008.090662. Epub 2008 Apr 17. Ann Rheum Dis. 2008. PMID: 18420938 Free PMC article.
-
Inflammatory regulation of glucocorticoid metabolism in mesenchymal stromal cells.Arthritis Rheum. 2012 Jul;64(7):2404-13. doi: 10.1002/art.34414. Arthritis Rheum. 2012. PMID: 22294469 Free PMC article.
-
Synergistic induction of local glucocorticoid generation by inflammatory cytokines and glucocorticoids: implications for inflammation associated bone loss.Ann Rheum Dis. 2010 Jun;69(6):1185-90. doi: 10.1136/ard.2009.107466. Epub 2009 Jun 22. Ann Rheum Dis. 2010. PMID: 19549618 Free PMC article.
-
Changing glucocorticoid action: 11β-hydroxysteroid dehydrogenase type 1 in acute and chronic inflammation.J Steroid Biochem Mol Biol. 2013 Sep;137:82-92. doi: 10.1016/j.jsbmb.2013.02.002. Epub 2013 Feb 19. J Steroid Biochem Mol Biol. 2013. PMID: 23435016 Free PMC article. Review.
-
Local amplification of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1 and its role in the inflammatory response.Ann N Y Acad Sci. 2006 Nov;1088:265-73. doi: 10.1196/annals.1366.030. Ann N Y Acad Sci. 2006. PMID: 17192572 Review.
Cited by
-
Transgenic disruption of endogenous glucocorticoid signaling in osteoblasts does not alter long-term K/BxN serum transfer-induced arthritis.Arthritis Res Ther. 2023 Aug 4;25(1):140. doi: 10.1186/s13075-023-03112-9. Arthritis Res Ther. 2023. PMID: 37542341 Free PMC article.
-
Functional analysis of fibroblasts and macrophages in head and neck paragangliomas.Front Endocrinol (Lausanne). 2024 Nov 15;15:1397839. doi: 10.3389/fendo.2024.1397839. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39619326 Free PMC article.
-
Glucocorticoid-induced osteoporosis - a disorder of mesenchymal stromal cells?Front Endocrinol (Lausanne). 2011 Aug 27;2:24. doi: 10.3389/fendo.2011.00024. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22654798 Free PMC article.
-
Glucocorticoids in bone and joint disease: the good, the bad and the uncertain.Clin Med (Lond). 2012 Jun;12(3):261-5. doi: 10.7861/clinmedicine.12-3-261. Clin Med (Lond). 2012. PMID: 22783780 Free PMC article.
-
Cortisol biosynthesis in the human ocular surface innate immune response.PLoS One. 2014 Apr 15;9(4):e94913. doi: 10.1371/journal.pone.0094913. eCollection 2014. PLoS One. 2014. PMID: 24736562 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

