Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages
- PMID: 16847070
- PMCID: PMC2118368
- DOI: 10.1084/jem.20060943
Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages
Abstract
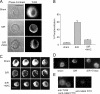
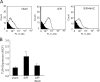
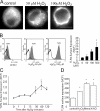
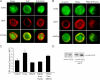
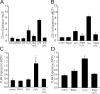
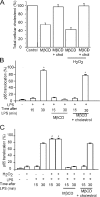
Oxidative stress generated by ischemia/reperfusion is known to prime inflammatory cells for increased responsiveness to subsequent stimuli, such as lipopolysaccharide (LPS). The mechanism(s) underlying this effect remains poorly elucidated. These studies show that alveolar macrophages recovered from rodents subjected to hemorrhagic shock/resuscitation expressed increased surface levels of Toll-like receptor 4 (TLR4), an effect inhibited by adding the antioxidant N-acetylcysteine to the resuscitation fluid. Consistent with a role for oxidative stress in this effect, in vitro H2O2 treatment of RAW 264.7 macrophages similarly caused an increase in surface TLR4. The H2O2-induced increase in surface TLR4 was prevented by depleting intracellular calcium or disrupting the cytoskeleton, suggesting the involvement of receptor exocytosis. Further, fluorescent resonance energy transfer between TLR4 and the raft marker GM1 as well as biochemical analysis of the raft components demonstrated that oxidative stress redistributes TLR4 to lipid rafts in the plasma membrane. Preventing the oxidant-induced movement of TLR4 to lipid rafts using methyl-beta-cyclodextrin precluded the increased responsiveness of cells to LPS after H2O2 treatment. Collectively, these studies suggest a novel mechanism whereby oxidative stress might prime the responsiveness of cells of the innate immune system.
Figures



References
-
- Sauaia, A., F.A. Moore, E.E. Moore, and D.C. Lezotte. 1996. Early risk factors for postinjury multiple organ failure. World J. Surg. 20:392–400. - PubMed
-
- Regel, G., M. Grotz, T. Weltner, J.A. Sturm, and H. Tscherne. 1996. Pattern of organ failure following severe trauma. World J. Surg. 20:422–429. - PubMed
-
- Botha, A.J., F.A. Moore, E.E. Moore, F.J. Kim, A. Banerjee, and V.M. Peterson. 1995. Postinjury neutrophil priming and activation: an early vulnerable window. Surgery. 118:358–364. - PubMed
-
- Moore, F.A., and E.E. Moore. 1995. Evolving concepts in the pathogenesis of postinjury multiple organ failure. Surg. Clin. North Am. 75:257–277. - PubMed
-
- Fan, J., J.C. Marshall, M. Jimenez, P.N. Shek, J. Zagorski, and O.D. Rotstein. 1998. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J. Immunol. 161:440–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

