Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling
- PMID: 16847102
- PMCID: PMC2064184
- DOI: 10.1083/jcb.200605028
Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling
Abstract
Adult skeletal muscle is able to repeatedly regenerate because of the presence of satellite cells, a population of stem cells resident beneath the basal lamina that surrounds each myofiber. Little is known, however, of the signaling pathways involved in the activation of satellite cells from quiescence to proliferation, a crucial step in muscle regeneration. We show that sphingosine-1-phosphate induces satellite cells to enter the cell cycle. Indeed, inhibiting the sphingolipid-signaling cascade that generates sphingosine-1-phosphate significantly reduces the number of satellite cells able to proliferate in response to mitogen stimulation in vitro and perturbs muscle regeneration in vivo. In addition, metabolism of sphingomyelin located in the inner leaflet of the plasma membrane is probably the main source of sphingosine-1-phosphate used to mediate the mitogenic signal. Together, our observations show that sphingolipid signaling is involved in the induction of proliferation in an adult stem cell and a key component of muscle regeneration.
Figures


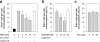

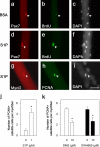
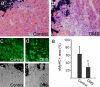
References
-
- Andrieu, N., R. Salvayre, and T. Levade. 1996. Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur. J. Biochem. 236:738–745. - PubMed
-
- Andrieu-Abadie, N., and T. Levade. 2002. Sphingomyelin hydrolysis during apoptosis. Biochim. Biophys. Acta. 1585:126–134. - PubMed
-
- Bischoff, R. 1986. Proliferation of muscle satellite cells on intact myofibers in culture. Dev. Biol. 115:129–139. - PubMed
-
- Chargé, S.B., and M.A. Rudnicki. 2004. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84:209–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

