Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage
- PMID: 16847263
- PMCID: PMC1544094
- DOI: 10.1073/pnas.0600768103
Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage
Abstract
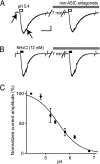
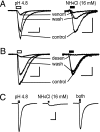
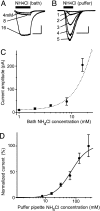
Acid-sensitive ion channels (ASICs) are proton-gated and belong to the family of degenerin channels. In the mammalian nervous system, ASICs are most well known in sensory neurons, where they are involved in nociception, occurring when injury or inflammation causes acidification. ASICs also are widely expressed in the CNS, and some synaptic roles have been revealed. Because neuronal activity can produce pH changes, ASICs may respond to local acidic transients and alter the excitability of neuronal circuits more widely than is presently appreciated. Furthermore, ASICs have been found to underlie calcium transients that contribute to neuronal death. Degeneration of midbrain dopamine neurons is characteristic of advanced idiopathic Parkinson's disease. Therefore, we tested for functional ASICs in midbrain dopamine neurons of the ventral tegmental area and substantia nigra compacta. Patch-clamp electrophysiology applied to murine midbrain slices revealed abundant acid-sensitive channels. The ASICs were gated and desensitized by extracellular application of millimolar concentrations of NH(4)Cl. Although the NH(4)Cl solution contains micromolar concentrations of NH(3) at pH 7.4, our evidence indicates that NH(4)(+) gates the ASICs. The proton-gated and the ammonium-gated currents were inhibited by tarantula venom (psalmotoxin), which is specific for the ASIC1a subtype. The results show that acid-sensitive channels are expressed in midbrain dopamine neurons and suggest that ammonium sensitivity is a widely distributed ASIC characteristic in the CNS, including the hippocampus. The ammonium sensitivity suggests a role for ASIC1s in hepatic encephalopathy, cirrhosis, and other neuronal disorders that are associated with hyperammonemia.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
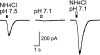

Similar articles
-
Functional characterization of acid-sensing ion channels in cultured neurons of rat inferior colliculus.Neuroscience. 2008 Jun 23;154(2):461-72. doi: 10.1016/j.neuroscience.2008.03.040. Epub 2008 Mar 26. Neuroscience. 2008. PMID: 18456416
-
The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice.Neuroscience. 2006 May 12;139(2):699-709. doi: 10.1016/j.neuroscience.2005.12.020. Epub 2006 Mar 3. Neuroscience. 2006. PMID: 16515841
-
Properties of the proton-evoked currents and their modulation by Ca2+ and Zn2+ in the acutely dissociated hippocampus CA1 neurons.Brain Res. 2004 Aug 13;1017(1-2):197-207. doi: 10.1016/j.brainres.2004.05.046. Brain Res. 2004. PMID: 15261115
-
Proton-gated cation channels--neuronal acid sensors in the central and peripheral nervous system.Adv Exp Med Biol. 2001;502:293-304. doi: 10.1007/978-1-4757-3401-0_19. Adv Exp Med Biol. 2001. PMID: 11950145 Review.
-
Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases.Curr Opin Pharmacol. 2008 Feb;8(1):25-32. doi: 10.1016/j.coph.2007.09.001. Epub 2007 Oct 22. Curr Opin Pharmacol. 2008. PMID: 17945532 Free PMC article. Review.
Cited by
-
Acid-sensing ion channels contribute to chemosensitivity of breathing-related neurons of the nucleus of the solitary tract.J Physiol. 2012 Oct 1;590(19):4761-75. doi: 10.1113/jphysiol.2012.232470. Epub 2012 Aug 13. J Physiol. 2012. PMID: 22890703 Free PMC article.
-
A synaptic DEG/ENaC ion channel mediates learning in C. elegans by facilitating dopamine signalling.EMBO J. 2008 Dec 17;27(24):3288-99. doi: 10.1038/emboj.2008.252. Epub 2008 Nov 27. EMBO J. 2008. PMID: 19037257 Free PMC article.
-
ASIC1a activation enhances inhibition in the basolateral amygdala and reduces anxiety.J Neurosci. 2014 Feb 26;34(9):3130-41. doi: 10.1523/JNEUROSCI.4009-13.2014. J Neurosci. 2014. PMID: 24573273 Free PMC article.
-
Loss-of-function and gain-of-function phenotypes of stomatocytosis mutant RhAG F65S.Am J Physiol Cell Physiol. 2011 Dec;301(6):C1325-43. doi: 10.1152/ajpcell.00054.2011. Epub 2011 Aug 17. Am J Physiol Cell Physiol. 2011. PMID: 21849667 Free PMC article.
-
Parkin-mediated monoubiquitination of the PDZ protein PICK1 regulates the activity of acid-sensing ion channels.Mol Biol Cell. 2007 Aug;18(8):3105-18. doi: 10.1091/mbc.e05-11-1027. Epub 2007 Jun 6. Mol Biol Cell. 2007. PMID: 17553932 Free PMC article.
References
-
- Krishtal O. A., Pidoplichko V. I. Neuroscience. 1980;5:2325–2327. - PubMed
-
- Krishtal O. A., Pidoplichko V. I. Neuroscience. 1981;6:2599–2601. - PubMed
-
- Krishtal O. A., Pidoplichko V. I. Neurosci. Lett. 1981;24:243–246. - PubMed
-
- McCleskey E. W., Gold M. S. Annu. Rev. Physiol. 1999;61:835–856. - PubMed
-
- Waldmann R. Adv. Exp. Med. Biol. 2001;502:293–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

