Role of human mitochondrial Nfs1 in cytosolic iron-sulfur protein biogenesis and iron regulation
- PMID: 16847322
- PMCID: PMC1592756
- DOI: 10.1128/MCB.00112-06
Role of human mitochondrial Nfs1 in cytosolic iron-sulfur protein biogenesis and iron regulation
Abstract
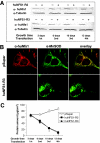
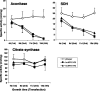
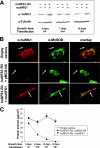
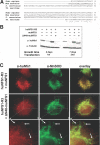
The biogenesis of iron-sulfur (Fe/S) proteins in eukaryotes is a complex process involving more than 20 components. So far, functional investigations have mainly been performed in Saccharomyces cerevisiae. Here, we have analyzed the role of the human cysteine desulfurase Nfs1 (huNfs1), which serves as a sulfur donor in biogenesis. The protein is located predominantly in mitochondria, but small amounts are present in the cytosol/nucleus. huNfs1 was depleted efficiently in HeLa cells by a small interfering RNA (siRNA) approach, resulting in a drastic growth retardation and striking morphological changes of mitochondria. The activities of both mitochondrial and cytosolic Fe/S proteins were strongly impaired, demonstrating that huNfs1 performs an essential function in Fe/S protein biogenesis in human cells. Expression of murine Nfs1 (muNfs1) in huNfs1-depleted cells restored both growth and Fe/S protein activities to wild-type levels, indicating the specificity of the siRNA depletion approach. No complementation of the growth retardation was observed, when muNfs1 was synthesized without its mitochondrial presequence. This extramitochondrial muNfs1 did not support maintenance of Fe/S protein activities, neither in the cytosol nor in mitochondria. In conclusion, our study shows that the essential huNfs1 is required inside mitochondria for efficient maturation of cellular Fe/S proteins. The results have implications for the regulation of iron homeostasis by cytosolic iron regulatory protein 1.
Figures




Similar articles
-
The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria.EMBO J. 2006 Jan 11;25(1):174-83. doi: 10.1038/sj.emboj.7600905. Epub 2005 Dec 8. EMBO J. 2006. PMID: 16341090 Free PMC article.
-
Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins.EMBO J. 2006 Jan 11;25(1):184-95. doi: 10.1038/sj.emboj.7600906. Epub 2005 Dec 8. EMBO J. 2006. PMID: 16341089 Free PMC article.
-
Functional characterization of the eukaryotic cysteine desulfurase Nfs1p from Saccharomyces cerevisiae.J Biol Chem. 2004 Aug 27;279(35):36906-15. doi: 10.1074/jbc.M406516200. Epub 2004 Jun 25. J Biol Chem. 2004. PMID: 15220327
-
The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism.Biochim Biophys Acta. 2012 Sep;1823(9):1491-508. doi: 10.1016/j.bbamcr.2012.05.009. Epub 2012 May 15. Biochim Biophys Acta. 2012. PMID: 22609301 Review.
-
Mitochondrial iron-sulfur protein biogenesis and human disease.Biochimie. 2014 May;100:61-77. doi: 10.1016/j.biochi.2014.01.010. Epub 2014 Jan 23. Biochimie. 2014. PMID: 24462711 Review.
Cited by
-
Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors.Hum Mol Genet. 2013 Dec 15;22(24):4871-87. doi: 10.1093/hmg/ddt338. Epub 2013 Jul 12. Hum Mol Genet. 2013. PMID: 23851121 Free PMC article.
-
Loss of Mitochondrial Localization of Human FANCG Causes Defective FANCJ Helicase.Mol Cell Biol. 2020 Nov 6;40(23):e00306-20. doi: 10.1128/MCB.00306-20. Print 2020 Nov 6. Mol Cell Biol. 2020. PMID: 32989015 Free PMC article.
-
NIF-type iron-sulfur cluster assembly system is duplicated and distributed in the mitochondria and cytosol of Mastigamoeba balamuthi.Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7371-6. doi: 10.1073/pnas.1219590110. Epub 2013 Apr 15. Proc Natl Acad Sci U S A. 2013. PMID: 23589868 Free PMC article.
-
Defects in the Maturation of Mitochondrial Iron-Sulfur Proteins: Biophysical Investigation of the MMDS3 Causing Gly104Cys Variant of IBA57.Int J Mol Sci. 2024 Sep 28;25(19):10466. doi: 10.3390/ijms251910466. Int J Mol Sci. 2024. PMID: 39408793 Free PMC article.
-
Human ind1, an iron-sulfur cluster assembly factor for respiratory complex I.Mol Cell Biol. 2009 Nov;29(22):6059-73. doi: 10.1128/MCB.00817-09. Epub 2009 Sep 14. Mol Cell Biol. 2009. PMID: 19752196 Free PMC article.
References
-
- Acquaviva, F., I. De Biase, L. Nezi, G. Ruggiero, F. Tatangelo, C. Pisano, A. Monticelli, C. Garbi, A. M. Acquaviva, and S. Cocozza. 2005. Extra-mitochondrial localisation of frataxin and its association with IscU1 during enterocyte-like differentiation of the human colon adenocarcinoma cell line Caco-2. J. Cell Sci. 118:3917-3924. - PubMed
-
- Amarzguioui, M., J. J. Rossi, and D. Kim. 2005. Approaches for chemically synthesized siRNA and vector-mediated RNAi. FEBS Lett. 579:5974-5981. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
