MafB is essential for renal development and F4/80 expression in macrophages
- PMID: 16847325
- PMCID: PMC1592773
- DOI: 10.1128/MCB.00001-06
MafB is essential for renal development and F4/80 expression in macrophages
Abstract
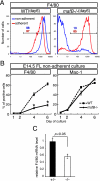
MafB is a member of the large Maf family of transcription factors that share similar basic region/leucine zipper DNA binding motifs and N-terminal activation domains. Although it is well known that MafB is specifically expressed in glomerular epithelial cells (podocytes) and macrophages, characterization of the null mutant phenotype in these tissues has not been previously reported. To investigate suspected MafB functions in the kidney and in macrophages, we generated mafB/green fluorescent protein (GFP) knock-in null mutant mice. MafB homozygous mutants displayed renal dysgenesis with abnormal podocyte differentiation as well as tubular apoptosis. Interestingly, these kidney phenotypes were associated with diminished expression of several kidney disease-related genes. In hematopoietic cells, GFP fluorescence was observed in both Mac-1- and F4/80-expressing macrophages in the fetal liver. Interestingly, F4/80 expression in macrophages was suppressed in the homozygous mutant, although development of the Mac-1-positive macrophage population was unaffected. In primary cultures of fetal liver hematopoietic cells, MafB deficiency was found to dramatically suppress F4/80 expression in nonadherent macrophages, whereas the Mac-1-positive macrophage population developed normally. These results demonstrate that MafB is essential for podocyte differentiation, renal tubule survival, and F4/80 maturation in a distinct subpopulation of nonadherent mature macrophages.
Figures







References
-
- Abrahamson, D. 1991. Glomerulogenesis in the developing kidney. Semin. Nephrol. 11:375-389. - PubMed
-
- Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11:805-815. - PubMed
-
- Barletta, G., I. A. Kovari, R. K. Verma, D. Kerjaschkil, and L. B. Holzman. 2003. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J. Biol. Chem. 278:19266-19271. - PubMed
-
- Blanchi. B., L. M. Kelly, J. C. Viemari, I. Lafon, H. Burnet, M. Bevengut, S. Tillmanns, L. Daniel, T. Graf, G. Hilaire, and M. H. Sieweke. 2003. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat. Neurosci. 10:1091-1100. - PubMed
-
- Boute, N., O. Gribouval, S. Roselli, F. Benessy, H. Lee, A. Fuchshuber, K. Dahan, M. C. Gubler, P. Niaudet, and C. Antignac. 2000. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24:349-354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
