MyoD synergizes with the E-protein HEB beta to induce myogenic differentiation
- PMID: 16847330
- PMCID: PMC1592768
- DOI: 10.1128/MCB.02404-05
MyoD synergizes with the E-protein HEB beta to induce myogenic differentiation
Erratum in
- Mol Cell Biol. 2013 Nov;33(21):4357
Abstract
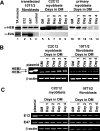
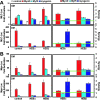
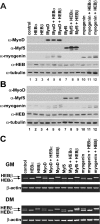
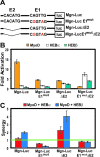
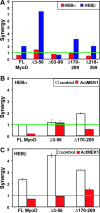
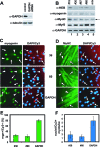
The MyoD family of basic helix-loop-helix transcription factors function as heterodimers with members of the E-protein family to induce myogenic gene activation. The E-protein HEB is alternatively spliced to generate alpha and beta isoforms. While the function of these molecules has been studied in other cell types, questions persist regarding the molecular functions of HEB proteins in skeletal muscle. Our data demonstrate that HEB alpha expression remains unchanged in both myoblasts and myotubes, whereas HEB beta is upregulated during the early phases of terminal differentiation. Upon induction of differentiation, a MyoD-HEB beta complex bound the E1 E-box of the myogenin promoter leading to transcriptional activation. Importantly, forced expression of HEB beta with MyoD synergistically lead to precocious myogenin expression in proliferating myoblasts. However, after differentiation, HEB alpha and HEB beta synergized with myogenin, but not MyoD, to activate the myogenin promoter. Specific knockdown of HEB beta by small interfering RNA in myoblasts blocked differentiation and inhibited induction of myogenin transcription. Therefore, HEB alpha and HEB beta play novel and central roles in orchestrating the regulation of myogenic factor activity through myogenic differentiation.
Figures
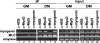
References
-
- Ballinger, C. A., P. Connell, Y. Wu, Z. Hu, L. J. Thompson, L. Y. Yin, and C. Patterson. 1999. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 19:4535-4545. - PMC - PubMed
-
- Bergstrom, D. A., B. H. Penn, A. Strand, R. L. Perry, M. A. Rudnicki, and S. J. Tapscott. 2002. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9:587-600. - PubMed
-
- Berkes, C. A., D. A. Bergstrom, B. H. Penn, K. J. Seaver, P. S. Knoepfler, and S. J. Tapscott. 2004. Pbx marks genes for activation by MyoD indicating a role for a homeodomain protein in establishing myogenic potential. Mol. Cell 14:465-477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
