Txr1: a transcriptional regulator of thrombospondin-1 that modulates cellular sensitivity to taxanes
- PMID: 16847352
- PMCID: PMC1536059
- DOI: 10.1101/gad.1441306
Txr1: a transcriptional regulator of thrombospondin-1 that modulates cellular sensitivity to taxanes
Abstract
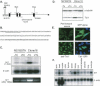
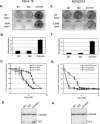
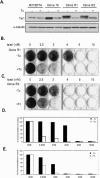
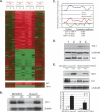
Using transcripts initiated at a chromosomally integrated retrovirus-based promoter to perturb gene expression randomly in human prostate cancer cells, we isolated cell clones resistant to taxane lethality and discovered the role of a previously uncharacterized gene, txr1, in this phenotype. We show that txr1 impedes taxane-induced apoptosis in tumor cells by transcriptionally down-regulating the production of thrombospondin-1 (TSP-1)--known earlier for both its anti-angiogenic and proapoptotic actions. Decrease of Txr1 or treatment with TSP-1 or TSP-1 mimetic peptide sensitized cells to taxane cytotoxicity by activating signaling through the CD47 receptor (also known as the integrin-associated protein), whereas interference with CD47 function reduced taxane-induced cell death. Cellular abundance of Txr1 and TSP-1 varied inversely, and alteration of the level of both proteins correlated highly with taxol resistance in 13 of 19 NCI-60 cancer cell lines. Our results reveal a hitherto unsuspected mechanism of taxane resistance, elucidate the role of txr1 in this resistance, and identify txr1 as a regulator of TSP-1 production and an agent for its chemotherapeutic modulation.
Figures

Similar articles
-
TXR1-mediated thrombospondin repression: a novel mechanism of resistance to taxanes?Genes Dev. 2006 Aug 1;20(15):1975-81. doi: 10.1101/gad.1460806. Genes Dev. 2006. PMID: 16882973 Review. No abstract available.
-
miR-130a activates apoptotic signaling through activation of caspase-8 in taxane-resistant prostate cancer cells.Prostate. 2015 Oct;75(14):1568-78. doi: 10.1002/pros.23031. Epub 2015 Jun 12. Prostate. 2015. PMID: 26074357
-
TXR1 and TSP1 expression varies by the molecular subtypes of breast cancer patients who received previous docetaxel-based first-line chemotherapy.Exp Biol Med (Maywood). 2016 Nov;241(17):1919-1923. doi: 10.1177/1535370216657443. Epub 2016 Jul 12. Exp Biol Med (Maywood). 2016. PMID: 27407097 Free PMC article.
-
Development of taxane resistance in a panel of human lung cancer cell lines.Toxicol In Vitro. 2008 Aug;22(5):1234-41. doi: 10.1016/j.tiv.2008.04.005. Epub 2008 Apr 15. Toxicol In Vitro. 2008. PMID: 18514476
-
Taxane resistance in breast cancer: mechanisms, predictive biomarkers and circumvention strategies.Cancer Treat Rev. 2012 Nov;38(7):890-903. doi: 10.1016/j.ctrv.2012.02.011. Epub 2012 Mar 31. Cancer Treat Rev. 2012. PMID: 22465195 Review.
Cited by
-
Thrombospondin-1 as a Paradigm for the Development of Antiangiogenic Agents Endowed with Multiple Mechanisms of Action.Pharmaceuticals (Basel). 2010 Apr 23;3(4):1241-1278. doi: 10.3390/ph3041241. Pharmaceuticals (Basel). 2010. PMID: 27713299 Free PMC article. Review.
-
Txr1: an important factor in oxaliplatin resistance in gastric cancer.Med Oncol. 2014 Feb;31(2):807. doi: 10.1007/s12032-013-0807-1. Epub 2013 Dec 21. Med Oncol. 2014. PMID: 24362794
-
Alternative polyadenylation-related genetic variants contribute to bladder cancer risk.J Biomed Res. 2023 Nov 15;37(6):405-417. doi: 10.7555/JBR.37.20230063. J Biomed Res. 2023. PMID: 37936490 Free PMC article.
-
Early transcriptional response in the jejunum of germ-free piglets after oral infection with virulent rotavirus.Arch Virol. 2008;153(7):1311-22. doi: 10.1007/s00705-008-0118-6. Epub 2008 Jun 4. Arch Virol. 2008. PMID: 18523839 Free PMC article.
-
Armed Oncolytic Adenovirus-Expressing PD-L1 Mini-Body Enhances Antitumor Effects of Chimeric Antigen Receptor T Cells in Solid Tumors.Cancer Res. 2017 Apr 15;77(8):2040-2051. doi: 10.1158/0008-5472.CAN-16-1577. Epub 2017 Feb 24. Cancer Res. 2017. PMID: 28235763 Free PMC article.
References
-
- Bae V.L., Jackson-Cook C.K., Maygarden S.J., Plymate S.R., Chen J., Ware J.L. Metastatic sublines of an SV40 large T antigen immortalized human prostate epithelial cell line. Prostate. 1998;34:275–282. - PubMed
-
- Berns K., Hijmans E.M., Mullenders J., Brummelkamp T.R., Velds A., Heimerikx M., Kerkhoven R.M., Madiredjo M., Nijkamp W., Weigelt B., et al. A large-scale RNAi screen in human cells identifies new components of the p53 pathway. Nature. 2004;428:431–437. - PubMed
-
- Bradley G., Ling V. P-Glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994;13:223–233. - PubMed
-
- Brown E.J., Frazier W.A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
