Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice
- PMID: 16847440
- PMCID: PMC1752018
- DOI: 10.1038/sj.bjp.0706824
Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice
Erratum in
- Br J Pharmacol. 2007 Jan;150(2):249. Basavarajppa, Balapal S [corrected to Basavarajappa, Balapal S]
Abstract
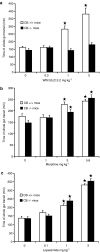
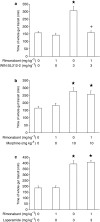
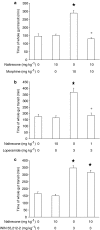
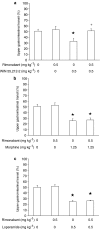
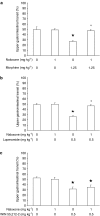
1. This study investigated whether (a) cannabinoid CB(1) receptor knockout (CB(1)(-/-)) mice displayed altered gastrointestinal transit and (b) cannabinoid CB(1) and opioid receptors functionally interact in the regulation of gastrointestinal transit. 2. Gastrointestinal transit was assessed by the Whole Gastrointestinal Transit, measuring the excretion time of an intragastrically administered marker (whole intestine), and the Upper Gastrointestinal Transit, measuring the distance covered by the marker in the small intestine. 3. CB(1)(-/-) and homozygous CB(1)(+/+) (CB(1)(+/+)) mice did not differ in both whole gut and small intestine transit. CB(1)(-/-) and CB(1)(+/+) mice were equally responsive to the inhibitory effect of morphine (10 mg kg(-1)) and loperamide (3 mg kg(-1)) on whole gut transit.4. Additionally, in CD1 mice the cannabinoid CB(1) receptor antagonist, rimonabant (0-0.5 mg kg(-1)), failed to block the inhibitory effect of morphine (0-1.25 mg kg(-1)) and loperamide (0-0.5 mg kg(-1)) on transit in small and whole intestine. Similarly, the opioid receptor antagonists, naloxone (0-1 mg kg(-1)) and naltrexone (0-10 mg kg(-1)), failed to block the inhibitory effect of the cannabinoid WIN 55,212-2 (0-3 mg kg(-1)) on transit in small and whole intestine.5. These results suggest that (a) compensatory mechanisms likely developed in CB(1)(-/-) mice to overcome the lack of inhibitory function of endocannabinoid system; (b) cannabinoid and opioid receptor systems did not interact in regulating gastrointestinal transit in mice.
Figures

Similar articles
-
Differential effects of CB(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice.Neurogastroenterol Motil. 2010 Jul;22(7):787-96, e223. doi: 10.1111/j.1365-2982.2010.01478.x. Epub 2010 Feb 24. Neurogastroenterol Motil. 2010. PMID: 20180825 Free PMC article.
-
Central administrations of hemopressin and related peptides inhibit gastrointestinal motility in mice.Neurogastroenterol Motil. 2016 Jun;28(6):891-9. doi: 10.1111/nmo.12789. Epub 2016 Mar 14. Neurogastroenterol Motil. 2016. PMID: 26991932
-
Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone (SAB378), a peripherally restricted cannabinoid CB1/CB2 receptor agonist, inhibits gastrointestinal motility but has no effect on experimental colitis in mice.J Pharmacol Exp Ther. 2010 Sep 1;334(3):973-80. doi: 10.1124/jpet.110.169946. Epub 2010 Jun 22. J Pharmacol Exp Ther. 2010. PMID: 20571060
-
Cannabinoids and the gastrointestinal tract.Gut. 2001 Jun;48(6):859-67. doi: 10.1136/gut.48.6.859. Gut. 2001. PMID: 11358910 Free PMC article. Review.
-
Role of opioid ligands in the irritable bowel syndrome.Can J Gastroenterol. 1999 Mar;13 Suppl A:71A-75A. doi: 10.1155/1999/598659. Can J Gastroenterol. 1999. PMID: 10202212 Review.
Cited by
-
Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice.Br J Pharmacol. 2008 Jul;154(5):1001-8. doi: 10.1038/bjp.2008.177. Epub 2008 May 12. Br J Pharmacol. 2008. PMID: 18469842 Free PMC article.
-
Negative allosteric modulation of CB1 cannabinoid receptor signaling suppresses opioid-mediated tolerance and withdrawal without blocking opioid antinociception.Neuropharmacology. 2024 Oct 1;257:110052. doi: 10.1016/j.neuropharm.2024.110052. Epub 2024 Jun 25. Neuropharmacology. 2024. PMID: 38936657 Free PMC article.
-
The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet.Br J Pharmacol. 2008 Mar;153(6):1272-80. doi: 10.1038/sj.bjp.0707682. Epub 2008 Jan 28. Br J Pharmacol. 2008. PMID: 18223666 Free PMC article.
-
Differential effects of CB(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice.Neurogastroenterol Motil. 2010 Jul;22(7):787-96, e223. doi: 10.1111/j.1365-2982.2010.01478.x. Epub 2010 Feb 24. Neurogastroenterol Motil. 2010. PMID: 20180825 Free PMC article.
-
A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents.Br J Pharmacol. 2010 Oct;161(3):629-42. doi: 10.1111/j.1476-5381.2010.00908.x. Br J Pharmacol. 2010. PMID: 20880401 Free PMC article.
References
-
- CALIGNANO A., LA RANA G., MAKRIYANNIS A., LIN S.Y., BELTRAMO M., PIOMELLI D. Inhibition of intestinal motility by anandamide, an endogenous cannabinoid. Eur. J. Pharmacol. 1997;340:R7–R8. - PubMed
-
- CAPASSO R., MATIAS I., LUTZ B., BORRELLI F., CAPASSO F., MORSICANO G., MASCOLO N., PETROSINO S., MONORY K., VALENTI M., DI MARZO V., IZZO A.A. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. - PubMed
-
- CARAI M.A.M., COLOMBO G., GESSA G.L. Rapid tolerance to the intestinal prokinetic effect of cannabinoid CB1 receptor antagonist, SR 141716 (rimonabant) Eur. J. Pharmacol. 2004;494:221–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

