Regio- and chemoselective covalent immobilization of proteins through unnatural amino acids
- PMID: 16848430
- PMCID: PMC2516943
- DOI: 10.1021/ja061131o
Regio- and chemoselective covalent immobilization of proteins through unnatural amino acids
Abstract
A general approach was developed for the regio- and chemoselective covalent immobilization of soluble proteins on glass surfaces through an unnatural amino acid created by post-translationally modifying the cysteine residue in a CaaX recognition motif with functional groups suitable for "click" chemistry or a Staudinger ligation. Farnesyl diphosphate analogues bearing omega-azide or omega-alkyne moieties were attached to the cysteine residue in Cys-Val-Ile-Ala motifs at the C-termini of engineered versions of green fluorescent protein (GFP) and glutathione S-transferase (GST) by protein farnesyltransferase. The derivatized proteins were attached to glass slides bearing linkers containing azide ("click" chemistry) or phosphine (Staudinger ligation) groups. "Click"-immobilized proteins were detected by fluorescently labeled antibodies and remained attached to the slide through two cycles of stripping under stringent conditions at 80 degrees C. GFP immobilized by a Staudinger ligation was detected by directly imagining the GFP fluorophore over a period of 6 days. These methods for covalent immobilization of proteins should be generally applicable. CaaX recognition motifs can easily be appended to the C-terminus of a cloned protein by a simple modification of the corresponding gene, and virtually any soluble protein or peptide bearing a CaaX motif is a substrate for protein farnesyltransferase.
Figures
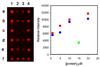
 ); GST proteins (
); GST proteins ( ); controls (
); controls ( ).
).
 ) immobilized by Staudinger ligation and the GFP-F (
) immobilized by Staudinger ligation and the GFP-F ( ) control after storage in buffer.
) control after storage in buffer.

References
-
- MacBeath G, Schreiber SL. Science. 2000;289:1760–1763. - PubMed
-
-
For recent reviews, see:Zhu H, Snyder M. Curr. Opin. Chem. Biol. 2003;7:55–63. Wilson DS, Nock S. Angew. Chem. Int. Ed. 2003;42:494–500. Ramachandran N. Curr. Opin. Chem. Biol. 2005;9:14–19.
-
-
- Zhu H, Bilgin M, Bangham R, Hall D, Casamayor A, Bertone P, Lan N, Jansen R, Bidlingmaier S, Houfek T, Mitchell T, Miller P, Dean RA, Gerstein M, Snyder M. Science. 2001;293:2101–2105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

