Complex parameter landscape for a complex neuron model
- PMID: 16848639
- PMCID: PMC1513272
- DOI: 10.1371/journal.pcbi.0020094
Complex parameter landscape for a complex neuron model
Abstract
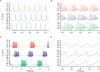
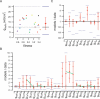
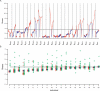
The electrical activity of a neuron is strongly dependent on the ionic channels present in its membrane. Modifying the maximal conductances from these channels can have a dramatic impact on neuron behavior. But the effect of such modifications can also be cancelled out by compensatory mechanisms among different channels. We used an evolution strategy with a fitness function based on phase-plane analysis to obtain 20 very different computational models of the cerebellar Purkinje cell. All these models produced very similar outputs to current injections, including tiny details of the complex firing pattern. These models were not completely isolated in the parameter space, but neither did they belong to a large continuum of good models that would exist if weak compensations between channels were sufficient. The parameter landscape of good models can best be described as a set of loosely connected hyperplanes. Our method is efficient in finding good models in this complex landscape. Unraveling the landscape is an important step towards the understanding of functional homeostasis of neurons.
Conflict of interest statement
Figures

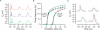
References
-
- MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron. 2003;37:109–120. - PubMed
-
- Schulz DJ, Goaillard JM, Marder E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci. 2006;9:356–362. - PubMed
-
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

