Macrophage pro-inflammatory response to Francisella novicida infection is regulated by SHIP
- PMID: 16848641
- PMCID: PMC1513262
- DOI: 10.1371/journal.ppat.0020071
Macrophage pro-inflammatory response to Francisella novicida infection is regulated by SHIP
Abstract
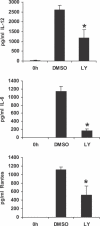
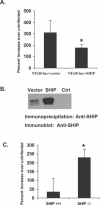
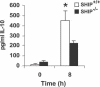
Francisella tularensis, a Gram-negative facultative intracellular pathogen infecting principally macrophages and monocytes, is the etiological agent of tularemia. Macrophage responses to F. tularensis infection include the production of pro-inflammatory cytokines such as interleukin (IL)-12, which is critical for immunity against infection. Molecular mechanisms regulating production of these inflammatory mediators are poorly understood. Herein we report that the SH2 domain-containing inositol phosphatase (SHIP) is phosphorylated upon infection of primary murine macrophages with the genetically related F. novicida, and negatively regulates F. novicida-induced cytokine production. Analyses of the molecular details revealed that in addition to activating the MAP kinases, F. novicida infection also activated the phosphatidylinositol 3-kinase (PI3K)/Akt pathway in these cells. Interestingly, SHIP-deficient macrophages displayed enhanced Akt activation upon F. novicida infection, suggesting elevated PI3K-dependent activation pathways in absence of SHIP. Inhibition of PI3K/Akt resulted in suppression of F. novicida-induced cytokine production through the inhibition of NFkappaB. Consistently, macrophages lacking SHIP displayed enhanced NFkappaB-driven gene transcription, whereas overexpression of SHIP led to decreased NFkappaB activation. Thus, we propose that SHIP negatively regulates F. novicida-induced inflammatory cytokine response by antagonizing the PI3K/Akt pathway and suppressing NFkappaB-mediated gene transcription. A detailed analysis of phosphoinositide signaling may provide valuable clues for better understanding the pathogenesis of tularemia.
Conflict of interest statement
Figures




References
-
- Santic M, Molmeret M, Klose KE, Jones S, Kwaik YA. The Francisella tularensis pathogenicity island protein IglC and its regulator MglA are essential for modulating phagosome biogenesis and subsequent bacterial escape into the cytoplasm. Cell Microbiol. 2005;7:969–979. - PubMed
-
- Lindgren H, Golovliov I, Baranov V, Ernst RK, Telepnev M, et al. Factors affecting the escape of Francisella tularensis from the phagolysosome. J Med Microbiol. 2004;53:953–958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

