Man2C1, an alpha-mannosidase, is involved in the trimming of free oligosaccharides in the cytosol
- PMID: 16848760
- PMCID: PMC1635433
- DOI: 10.1042/BJ20060945
Man2C1, an alpha-mannosidase, is involved in the trimming of free oligosaccharides in the cytosol
Abstract
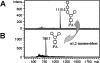
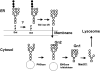
The endoplasmic-reticulum-associated degradation of misfolded (glyco)proteins ensures that only functional, correctly folded proteins exit from the endoplasmic reticulum and that misfolded ones are degraded by the ubiquitin-proteasome system. During the degradation of misfolded glycoproteins, they are deglycosylated by the PNGase (peptide:N-glycanase). The free oligosaccharides released by PNGase are known to be further catabolized by a cytosolic alpha-mannosidase, although the gene encoding this enzyme has not been identified unequivocally. The findings in the present study demonstrate that an alpha-mannosidase, Man2C1, is involved in the processing of free oligosaccharides that are formed in the cytosol. When the human Man2C1 orthologue was expressed in HEK-293 cells, most of the enzyme was localized in the cytosol. Its activity was enhanced by Co2+, typical of other known cytosolic alpha-mannosidases so far characterized from animal cells. The down-regulation of Man2C1 activity by a small interfering RNA drastically changed the amount and structure of oligosaccharides accumulating in the cytosol, demonstrating that Man2C1 indeed is involved in free oligosaccharide processing in the cytosol. The oligosaccharide processing in the cytosol by PNGase, endo-beta-N-acetylglucosaminidase and alpha-mannosidase may represent the common 'non-lysosomal' catabolic pathway for N-glycans in animal cells, although the molecular mechanism as well as the functional importance of such processes remains to be determined.
Figures



Similar articles
-
Structural diversity of cytosolic free oligosaccharides in the human hepatoma cell line, HepG2.Glycobiology. 2006 Apr;16(4):294-304. doi: 10.1093/glycob/cwj074. Epub 2005 Dec 27. Glycobiology. 2006. PMID: 16381657
-
Accumulation of free oligosaccharides and tissue damage in cytosolic α-mannosidase (Man2c1)-deficient mice.J Biol Chem. 2014 Apr 4;289(14):9611-22. doi: 10.1074/jbc.M114.550509. Epub 2014 Feb 18. J Biol Chem. 2014. PMID: 24550399 Free PMC article.
-
Calystegine B3 as a specific inhibitor for cytoplasmic alpha-mannosidase, Man2C1.J Biochem. 2011 Apr;149(4):415-22. doi: 10.1093/jb/mvq153. Epub 2011 Jan 8. J Biochem. 2011. PMID: 21217149
-
Mammalian alpha-mannosidases--multiple forms but a common purpose?Glycobiology. 1994 Oct;4(5):551-66. doi: 10.1093/glycob/4.5.551. Glycobiology. 1994. PMID: 7881169 Review.
-
Golgi alpha-mannosidase II deficiency in vertebrate systems: implications for asparagine-linked oligosaccharide processing in mammals.Biochim Biophys Acta. 2002 Dec 19;1573(3):225-35. doi: 10.1016/s0304-4165(02)00388-4. Biochim Biophys Acta. 2002. PMID: 12417404 Review.
Cited by
-
Iminosugar antivirals: the therapeutic sweet spot.Biochem Soc Trans. 2017 Apr 15;45(2):571-582. doi: 10.1042/BST20160182. Biochem Soc Trans. 2017. PMID: 28408497 Free PMC article. Review.
-
Cytosolic Free N-Glycans Are Retro-Transported Into the Endoplasmic Reticulum in Plant Cells.Front Plant Sci. 2021 Jan 18;11:610124. doi: 10.3389/fpls.2020.610124. eCollection 2020. Front Plant Sci. 2021. PMID: 33537045 Free PMC article.
-
N-glycan core tri-fucosylation requires Golgi α-mannosidase III activity that impacts nematode growth and behavior.J Biol Chem. 2024 Dec;300(12):107944. doi: 10.1016/j.jbc.2024.107944. Epub 2024 Oct 29. J Biol Chem. 2024. PMID: 39481603 Free PMC article.
-
Kex2 protease converts the endoplasmic reticulum alpha1,2-mannosidase of Candida albicans into a soluble cytosolic form.Microbiology (Reading). 2008 Dec;154(Pt 12):3782-3794. doi: 10.1099/mic.0.2008/019315-0. Microbiology (Reading). 2008. PMID: 19047746 Free PMC article.
-
Characterisation of class I and II α-mannosidases from Drosophila melanogaster.Glycoconj J. 2013 Dec;30(9):899-909. doi: 10.1007/s10719-013-9495-5. Epub 2013 Aug 25. Glycoconj J. 2013. PMID: 23979800
References
-
- Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. - PubMed
-
- Sayeed A., Ng D. T. Search and destroy: ER quality control and ER-associated protein degradation. Crit. Rev. Biochem. Mol. Biol. 2005;40:75–91. - PubMed
-
- Suzuki T., Park H., Lennarz W. J. Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure and potential functions. FASEB J. 2002;16:635–641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

