GroEL-mediated protein folding: making the impossible, possible
- PMID: 16849107
- PMCID: PMC3783267
- DOI: 10.1080/10409230600760382
GroEL-mediated protein folding: making the impossible, possible
Abstract
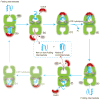
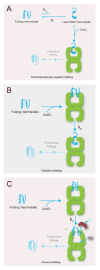
Protein folding is a spontaneous process that is essential for life, yet the concentrated and complex interior of a cell is an inherently hostile environment for the efficient folding of many proteins. Some proteins-constrained by sequence, topology, size, and function-simply cannot fold by themselves and are instead prone to misfolding and aggregation. This problem is so deeply entrenched that a specialized family of proteins, known as molecular chaperones, evolved to assist in protein folding. Here we examine one essential class of molecular chaperones, the large, oligomeric, and energy utilizing chaperonins or Hsp60s. The bacterial chaperonin GroEL, along with its co-chaperonin GroES, is probably the best-studied example of this family of protein-folding machine. In this review, we examine some of the general properties of proteins that do not fold well in the absence of GroEL and then consider how folding of these proteins is enhanced by GroEL and GroES. Recent experimental and theoretical studies suggest that chaperonins like GroEL and GroES employ a combination of protein isolation, unfolding, and conformational restriction to drive protein folding under conditions where it is otherwise not possible.
Figures



Similar articles
-
The oligomeric structure of GroEL/GroES is required for biologically significant chaperonin function in protein folding.Nat Struct Biol. 1998 Nov;5(11):977-85. doi: 10.1038/2952. Nat Struct Biol. 1998. PMID: 9808043
-
Co-expression of chaperonin GroEL/GroES enhances in vivo folding of yeast mitochondrial aconitase and alters the growth characteristics of Escherichia coli.Int J Biochem Cell Biol. 2006;38(11):1975-85. doi: 10.1016/j.biocel.2006.05.013. Epub 2006 Jun 2. Int J Biochem Cell Biol. 2006. PMID: 16822698
-
Protein folding assisted by the GroEL/GroES chaperonin system.Biochemistry (Mosc). 1998 Apr;63(4):374-81. Biochemistry (Mosc). 1998. PMID: 9556520 Review.
-
The GroEL-GroES Chaperonin Machine: A Nano-Cage for Protein Folding.Trends Biochem Sci. 2016 Jan;41(1):62-76. doi: 10.1016/j.tibs.2015.07.009. Epub 2015 Sep 25. Trends Biochem Sci. 2016. PMID: 26422689 Review.
-
Exploring the kinetic requirements for enhancement of protein folding rates in the GroEL cavity.J Mol Biol. 1999 Apr 2;287(3):627-44. doi: 10.1006/jmbi.1999.2591. J Mol Biol. 1999. PMID: 10092464
Cited by
-
Functional Subunits of Eukaryotic Chaperonin CCT/TRiC in Protein Folding.J Amino Acids. 2011;2011:843206. doi: 10.4061/2011/843206. Epub 2011 Jul 2. J Amino Acids. 2011. PMID: 22312474 Free PMC article.
-
Archaeal-like chaperonins in bacteria.Proc Natl Acad Sci U S A. 2010 Nov 23;107(47):20269-74. doi: 10.1073/pnas.1004783107. Epub 2010 Nov 5. Proc Natl Acad Sci U S A. 2010. PMID: 21057109 Free PMC article.
-
Legionella pneumophila requires polyamines for optimal intracellular growth.J Bacteriol. 2011 Sep;193(17):4346-60. doi: 10.1128/JB.01506-10. Epub 2011 Jul 8. J Bacteriol. 2011. PMID: 21742865 Free PMC article.
-
The C-terminal tails of the bacterial chaperonin GroEL stimulate protein folding by directly altering the conformation of a substrate protein.J Biol Chem. 2014 Aug 15;289(33):23219-23232. doi: 10.1074/jbc.M114.577205. Epub 2014 Jun 25. J Biol Chem. 2014. PMID: 24970895 Free PMC article.
-
Protein folding at single-molecule resolution.Biochim Biophys Acta. 2011 Aug;1814(8):1021-9. doi: 10.1016/j.bbapap.2011.01.011. Epub 2011 Feb 17. Biochim Biophys Acta. 2011. PMID: 21303706 Free PMC article. Review.
References
-
- Agard DA. To fold or not to fold. Science. 1993;260:1903–1904. - PubMed
-
- Anfinsen CB. Principles that govern the folding of protein chains. Science. 1973;181:223–230. - PubMed
-
- Aoki K, Taguchi H, et al. Calorimetric observation of a GroEL-protein binding reaction with little contribution of hydrophobic interaction. J Biol Chem. 1997;272:32158–32162. - PubMed
-
- Ashcroft AE, Brinker A, et al. Structural plasticity and noncovalent substrate binding in the GroEL apical domain. A study using electrospay ionization mass spectrometry and fluorescence binding studies. J Biol Chem. 2002;277:33115–33126. - PubMed
-
- Badcoe IG, Smith CJ, et al. Binding of a chaperonin to the folding intermediates of lactate dehydrogenase. Biochemistry. 1991;30:9195–9200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
