Targeting protein-protein interactions by rational design: mimicry of protein surfaces
- PMID: 16849232
- PMCID: PMC1578744
- DOI: 10.1098/rsif.2006.0115
Targeting protein-protein interactions by rational design: mimicry of protein surfaces
Abstract
Protein-protein interactions play key roles in a range of biological processes, and are therefore important targets for the design of novel therapeutics. Unlike in the design of enzyme active site inhibitors, the disruption of protein-protein interactions is far more challenging, due to such factors as the large interfacial areas involved and the relatively flat and featureless topologies of these surfaces. Nevertheless, in spite of such challenges, there has been considerable progress in recent years. In this review, we discuss this progress in the context of mimicry of protein surfaces: targeting protein-protein interactions by rational design.
Figures

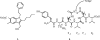
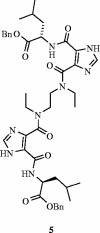
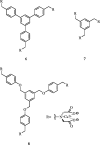
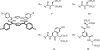
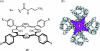
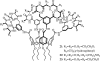
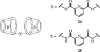
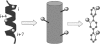
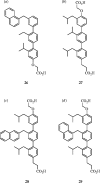
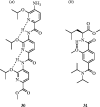
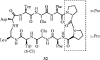
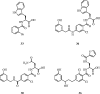
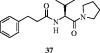
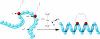

References
-
- Adler M, Carter P, Lazarus R.A, Wagner G. Cysteine pairing in the glycoprotein IIbIIIa antagonist kistrin using NMR, chemical analysis, and structure calculations. Biochemistry. 1993;32:282–289. doi:10.1021/bi00052a036 - DOI - PubMed
-
- Anderson K.V. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol. 2000;12:13–19. doi:10.1016/S0952-7915(99)00045-X - DOI - PubMed
-
- Anderson M.E, Yakovleva T, Hu Y, Siahaan T.J. Inhibition of ICAM-1/LFA-1-mediated heterotypic T-cell adhesion to epithelial cells: design of ICAM-1 cyclic peptides. Bioorg. Med. Chem. Lett. 2004;14:1399–1402. doi:10.1016/j.bmcl.2003.09.100 - DOI - PubMed
-
- Arkin M.R, Wells J.A. Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nat. Rev. Drug Discov. 2004;3:301–317. doi:10.1038/nrd1343 - DOI - PubMed
-
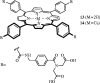
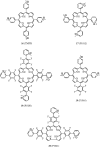
- Aviezer D, Cotton S, David M, Segev A, Khaselev N, Galili N, Gross Z, Yayon A. Porphyrin analogues as novel antagonists of fibroblast growth factor and vascular endothelial growth factor receptor binding that inhibit endothelial cell proliferation, tumor progression, and metastasis. Cancer Res. 2000;60:2973–2980. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
