Interfering with interferons in inflammatory bowel disease
- PMID: 16849343
- PMCID: PMC1856258
- DOI: 10.1136/gut.2005.090134
Interfering with interferons in inflammatory bowel disease
Abstract
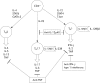
Early experience of fontolizumab, a humanised anti‐interferon γ antibody, in active Crohn's disease has shown that the drug caused a significant decrease in endoscopic severity scores and CRP and was reasonably well tolerated. Fontolizumab may be worthy of further clinical trials
Conflict of interest statement
Conflict of interest: None declared.
Comment on
-
A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn's disease.Gut. 2006 Aug;55(8):1138-44. doi: 10.1136/gut.2005.079434. Epub 2006 Feb 21. Gut. 2006. PMID: 16492717 Free PMC article. Clinical Trial.
-
Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease.Gut. 2006 Aug;55(8):1131-7. doi: 10.1136/gut.2005.079392. Epub 2006 Feb 28. Gut. 2006. PMID: 16507585 Free PMC article. Clinical Trial.
References
-
- Panitch H S, Folus J S, Johnson K P. Recombinant IFN‐β inhibits IFN‐gamma production in MS. Ann Neurol 198722137
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous