Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness
- PMID: 16849428
- PMCID: PMC1544057
- DOI: 10.1073/pnas.0603672103
Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness
Abstract
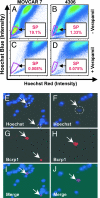
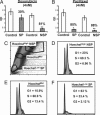
The recent identification of "side population" (SP) cells in a number of unrelated human cancers and their normal tissue sources has renewed interest in the hypothesis that cancers may arise from somatic stem/progenitor cells. The high incidence of recurrence attributable to multidrug resistance and the multiple histologic phenotypes indicative of multipotency suggests a stem cell-like etiology of ovarian cancer. Here we identify and characterize SP cells from two distinct genetically engineered mouse ovarian cancer cell lines. Differential efflux of the DNA-binding dye Hoechst 33342 from these cell lines defined a human breast cancer-resistance protein 1-expressing, verapamil-sensitive SP of candidate cancer stem cells. In vivo, mouse SP cells formed measurable tumors sooner than non-SP (NSP) cells when equal numbers were injected into the dorsal fat pad of nude mice. The presence of Mullerian Inhibiting Substance (MIS) signaling pathway transduction molecules in both SP and NSP mouse cells led us to investigate the efficacy of MIS against these populations in comparison with traditional chemotherapies. MIS inhibited the proliferation of both SP and NSP cells, whereas the lipophilic chemotherapeutic agent doxorubicin more significantly inhibited the NSP cells. Finally, we identified breast cancer-resistance protein 1-expressing verapamil-sensitive SPs in three of four human ovarian cancer cell lines and four of six patient primary ascites cells. In the future, individualized therapy must incorporate analysis of the stem cell-like subpopulation of ovarian cancer cells when designing therapeutic strategies for ovarian cancer patients.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


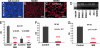

References
-
- Haraguchi N., Utsunomiya T., Inoue H., Tanaka F., Mimori K., Barnard G. F., Mori M. Stem Cells (Dayton, Ohio) 2006;24:506–513. - PubMed
-
- Wulf G. G., Wang R. Y., Kuehnle I., Weidner D., Marini F., Brenner M. K., Andreeff M., Goodell M. A. Blood. 2001;98:1166–1173. - PubMed
-
- Seigel G. M., Campbell L. M., Narayan M., Gonzalez-Fernandez F. Mol. Vis. 2005;11:729–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

