Barttin modulates trafficking and function of ClC-K channels
- PMID: 16849430
- PMCID: PMC1544099
- DOI: 10.1073/pnas.0601631103
Barttin modulates trafficking and function of ClC-K channels
Abstract
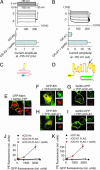
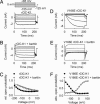
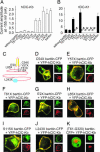
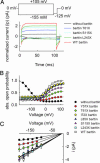
Barttin is an accessory subunit of a subgroup of ClC-type chloride channels expressed in renal and inner ear epithelia. In this study, we examined the effects of barttin on two ClC-K channel isoforms, rat ClC-K1 and human ClC-Kb, using heterologous expression, patch clamping, confocal imaging, and flow cytometry. In the absence of barttin, only a small percentage of rClC-K1 and hClC-Kb channels are inserted into the plasma membrane. Coexpression of barttin enhances surface membrane insertion and furthermore modifies permeation and gating of ClC-K channels. hClC-Kb channels are nonfunctional without barttin and require the coexpressed accessory subunit to become anion conducting. In contrast, rClC-K1 channels are active without barttin, but at the cost of reduced unitary conductance as well as altered voltage dependence of activation. We mapped the separate functions of barttin to structural domains by a deletion analysis. Whereas the transmembrane core is necessary and sufficient to promote ClC-K channel exit from the endoplasmic reticulum, a short cytoplasmic segment following the second transmembrane helix modifies the unitary conductance. The entire cytoplasmic carboxyl terminus affects the open probability of ClC-K channels. The multiple functions of barttin might be necessary for a tight adjustment of epithelial Cl(-) conductances to ensure a precise regulation of body salt content and endocochlear potential.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Hebert S. C. Curr. Opin. Nephrol. Hypertens. 2003;12:527–532. - PubMed
-
- Birkenhager R., Otto E., Schurmann M. J., Vollmer M., Ruf E. M., Maier-Lutz I., Beekmann F., Fekete A., Omran H., Feldmann D., et al. Nat. Genet. 2001;29:310–314. - PubMed
-
- Estevez R., Boettger T., Stein V., Birkenhager R., Otto E., Hildebrandt F., Jentsch T. J. Nature. 2001;414:558–561. - PubMed
-
- Waldegger S., Jeck N., Barth P., Peters M., Vitzthum H., Wolf K., Kurtz A., Konrad M., Seyberth H. W. Pflügers Arch. 2002;444:411–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

