Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision
- PMID: 16849433
- PMCID: PMC1544098
- DOI: 10.1073/pnas.0506112103
Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision
Abstract
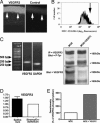
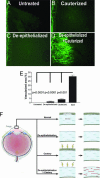
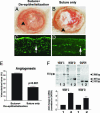
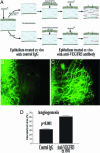
Transparency of the cornea, the window of the eye, is a prerequisite for vision. Angiogenesis into the normally avascular cornea is incompatible with good vision and, therefore, the cornea is one of the few tissues in the human body where avascularity is actively maintained. Here, we provide evidence for a critical mechanism contributing to corneal avascularity. VEGF receptor 3, normally present on lymphatic and proliferating blood vascular endothelium, is strongly constitutively expressed by corneal epithelium and is mechanistically responsible for suppressing inflammatory corneal angiogenesis.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Streilein J. W. Nat. Rev. Immunol. 2003;3:879–889. - PubMed
-
- Cursiefen C., Masli S., Ng T. F., Dana M. R., Bornstein P., Lawler J., Streilein J. W. Invest. Ophthalmol. Visual Sci. 2004;45:1117–1124. - PubMed
-
- Cursiefen C., Chen L., Dana M. R., Streilein J. W. Cornea. 2003;22:273–281. - PubMed
-
- Gimbrone M. A., Cotran R. S., Leapman S. B., Folkman J. J. Natl. Cancer Inst. 1974;52:413–427. - PubMed
-
- Kenyon B. M., Voest E. E., Chen C. C., Flynn E., Folkman J., D’Amato R. J. Invest. Ophthalmol. Visual Sci. 1996;37:1625–1632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

