Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium
- PMID: 16849458
- PMCID: PMC4015949
- DOI: 10.4049/jimmunol.177.3.1516
Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium
Abstract
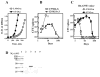
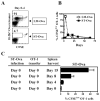
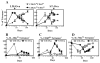
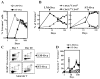
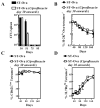
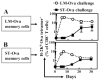
Ag presentation to CD8(+) T cells often commences immediately after infection, which facilitates their rapid expansion and control of infection. Subsequently, the primed cells undergo rapid contraction. We report that this paradigm is not followed during infection with virulent Salmonella enterica, serovar Typhimurium (ST), an intracellular bacterium that replicates within phagosomes of infected cells. Although susceptible mice die rapidly (approximately 7 days), resistant mice (129 x 1SvJ) harbor a chronic infection lasting approximately 60-90 days. Using rOVA-expressing ST (ST-OVA), we show that T cell priming is considerably delayed in the resistant mice. CD8(+) T cells that are induced during ST-OVA infection undergo delayed expansion, which peaks around day 21, and is followed by protracted contraction. Initially, ST-OVA induces a small population of cycling central phenotype (CD62L(high)IL-7Ralpha(high)CD44(high)) CD8(+) T cells. However, by day 14-21, majority of the primed CD8(+) T cells display an effector phenotype (CD62L(low)IL-7Ralpha(low)CD44(high)). Subsequently, a progressive increase in the numbers of effector memory phenotype cells (CD62L(low)IL-7Ralpha(high)CD44(high)) occurs. This differentiation program remained unchanged after accelerated removal of the pathogen with antibiotics, as majority of the primed cells displayed an effector memory phenotype even at 6 mo postinfection. Despite the chronic infection, CD8(+) T cells induced by ST-OVA were functional as they exhibited killing ability and cytokine production. Importantly, even memory CD8(+) T cells failed to undergo rapid expansion in response to ST-OVA infection, suggesting a delay in T cell priming during infection with virulent ST-OVA. Thus, phagosomal lifestyle may allow escape from host CD8(+) T cell recognition, conferring a survival advantage to the pathogen.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

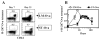
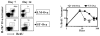


Similar articles
-
Intracellular bacterial vectors that induce CD8(+) T cells with similar cytolytic abilities but disparate memory phenotypes provide contrasting tumor protection.Cancer Res. 2009 May 15;69(10):4327-34. doi: 10.1158/0008-5472.CAN-08-3160. Epub 2009 May 12. Cancer Res. 2009. PMID: 19435919
-
Selectively reduced intracellular proliferation of Salmonella enterica serovar typhimurium within APCs limits antigen presentation and development of a rapid CD8 T cell response.J Immunol. 2009 Sep 15;183(6):3778-87. doi: 10.4049/jimmunol.0900843. Epub 2009 Aug 19. J Immunol. 2009. PMID: 19692639 Free PMC article.
-
Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2.J Immunol. 2007 Feb 15;178(4):2396-406. doi: 10.4049/jimmunol.178.4.2396. J Immunol. 2007. PMID: 17277146
-
Tracking the dynamics of salmonella specific T cell responses.Curr Top Microbiol Immunol. 2009;334:179-98. doi: 10.1007/978-3-540-93864-4_8. Curr Top Microbiol Immunol. 2009. PMID: 19521686 Free PMC article. Review.
-
Uncoupling protein 2 regulates metabolic reprogramming and fate of antigen-stimulated CD8+ T cells.Cancer Immunol Immunother. 2016 Jul;65(7):869-74. doi: 10.1007/s00262-016-1851-4. Epub 2016 Jun 6. Cancer Immunol Immunother. 2016. PMID: 27271549 Free PMC article. Review.
Cited by
-
MHC class-I-restricted CD8 T cells play a protective role during primary Salmonella infection.Immunol Lett. 2012 Dec 17;148(2):138-43. doi: 10.1016/j.imlet.2012.10.009. Epub 2012 Oct 23. Immunol Lett. 2012. PMID: 23089550 Free PMC article.
-
L-asparaginase II produced by Salmonella typhimurium inhibits T cell responses and mediates virulence.Cell Host Microbe. 2012 Dec 13;12(6):791-8. doi: 10.1016/j.chom.2012.10.018. Cell Host Microbe. 2012. PMID: 23245323 Free PMC article.
-
Salmonella enterica serovar Typhimurium-induced placental inflammation and not bacterial burden correlates with pathology and fatal maternal disease.Infect Immun. 2010 May;78(5):2292-301. doi: 10.1128/IAI.01186-09. Epub 2010 Mar 1. Infect Immun. 2010. PMID: 20194592 Free PMC article.
-
Early eradication of persistent Salmonella infection primes antibody-mediated protective immunity to recurrent infection.Microbes Infect. 2011 Apr;13(4):322-30. doi: 10.1016/j.micinf.2010.11.004. Epub 2010 Dec 4. Microbes Infect. 2011. PMID: 21134485 Free PMC article.
-
Salmonella enterica serovar Typhimurium lacking hfq gene confers protective immunity against murine typhoid.PLoS One. 2011 Feb 9;6(2):e16667. doi: 10.1371/journal.pone.0016667. PLoS One. 2011. PMID: 21347426 Free PMC article.
References
-
- Van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. - PubMed
-
- Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. - PubMed
-
- Wong P, Pamer EG. Feedback regulation of pathogen-specific T cell priming. Immunity. 2003;18:499–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

