Maternal gametophytic baseless1 is required for development of the central cell and early endosperm patterning in maize (Zea mays)
- PMID: 16849604
- PMCID: PMC1569813
- DOI: 10.1534/genetics.106.059709
Maternal gametophytic baseless1 is required for development of the central cell and early endosperm patterning in maize (Zea mays)
Abstract
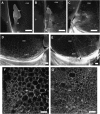
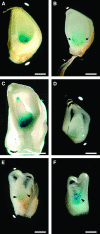
In angiosperms, double fertilization of an egg cell and a central cell with two sperm cells results in the formation of a seed containing a diploid embryo and a triploid endosperm. The extent to which the embryo sac controls postfertilization events in the seed is unknown. The novel gametophytic maternal-effect maize mutation, baseless1 (bsl1) affects central cell development within the embryo sac, frequently by altering the position of the two polar nuclei. Despite this irregularity, fertilization is as efficient as in wild type. The spatial expression of basal endosperm-specific transcripts is altered in free-nuclear and cellular mutant endosperms. At later stages of seed development, bsl1 predominantly affects development of the basal endosperm transfer layer (BETL). When bsl1/+ diploid plants were pollinated by wild-type tetraploid plants, the BETL abnormalities observed in bsl1/bsl1/+/+ tetraploid endosperms were diverse and of variable severity. Moreover, the frequency of kernels with severely perturbed BETL development correlated with the percentage of severely affected bsl1 central cells. Therefore, BSL1 is likely required in the central cell before fertilization for correct BETL patterning to occur. These findings provide new genetic evidence that a maternal gametophytic component is necessary for correct endosperm patterning.
Figures





Similar articles
-
Analysis of stunter1, a maize mutant with reduced gametophyte size and maternal effects on seed development.Genetics. 2011 Apr;187(4):1085-97. doi: 10.1534/genetics.110.125286. Epub 2011 Jan 26. Genetics. 2011. PMID: 21270392 Free PMC article.
-
A putative plant organelle RNA recognition protein gene is essential for maize kernel development.J Integr Plant Biol. 2015 Mar;57(3):236-46. doi: 10.1111/jipb.12234. Epub 2014 Aug 28. J Integr Plant Biol. 2015. PMID: 24985738
-
Maternal Gametophyte Effects on Seed Development in Maize.Genetics. 2016 Sep;204(1):233-48. doi: 10.1534/genetics.116.191833. Epub 2016 Jul 27. Genetics. 2016. PMID: 27466227 Free PMC article.
-
Genetic analysis as a tool to investigate the molecular mechanisms underlying seed development in maize.Ann Bot. 2005 Sep;96(3):353-62. doi: 10.1093/aob/mci187. Epub 2005 Jul 5. Ann Bot. 2005. PMID: 15998629 Free PMC article. Review.
-
Development and function of central cell in angiosperm female gametophyte.Genesis. 2010 Aug;48(8):466-78. doi: 10.1002/dvg.20647. Genesis. 2010. PMID: 20506265 Review.
Cited by
-
Intersection of transfer cells with phloem biology-broad evolutionary trends, function, and induction.Front Plant Sci. 2013 Jul 1;4:221. doi: 10.3389/fpls.2013.00221. eCollection 2013. Front Plant Sci. 2013. PMID: 23847631 Free PMC article.
-
Maize rough endosperm3 encodes an RNA splicing factor required for endosperm cell differentiation and has a nonautonomous effect on embryo development.Plant Cell. 2011 Dec;23(12):4280-97. doi: 10.1105/tpc.111.092163. Epub 2011 Dec 2. Plant Cell. 2011. PMID: 22138152 Free PMC article.
-
The Armadillo repeat gene ZAK IXIK promotes Arabidopsis early embryo and endosperm development through a distinctive gametophytic maternal effect.Plant Cell. 2012 Oct;24(10):4026-43. doi: 10.1105/tpc.112.102384. Epub 2012 Oct 12. Plant Cell. 2012. PMID: 23064319 Free PMC article.
-
Analysis of stunter1, a maize mutant with reduced gametophyte size and maternal effects on seed development.Genetics. 2011 Apr;187(4):1085-97. doi: 10.1534/genetics.110.125286. Epub 2011 Jan 26. Genetics. 2011. PMID: 21270392 Free PMC article.
-
Molecular mechanisms of maize endosperm transfer cell development.Plant Cell Rep. 2022 May;41(5):1171-1180. doi: 10.1007/s00299-021-02807-0. Epub 2021 Oct 24. Plant Cell Rep. 2022. PMID: 34689216 Review.
References
-
- Birchler, J. A., 1993. Dosage analysis of maize endosperm development. Annu. Rev. Genet. 27: 181–204. - PubMed
-
- Brown, R. C., B. E. Lemmon and H. Nguyen, 2003. Events during the first four rounds of mitosis establish three developmental domains in the syncytial endosperm of Arabidopsis thaliana. Protoplasma 222: 167–174. - PubMed
-
- Chang, M. T., and M. G. Neuffer, 1994. Endosperm-embryo interactions in maize. Maydica 39: 9–18.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

