Short-chain fatty acids induce gamma-globin gene expression by displacement of a HDAC3-NCoR repressor complex
- PMID: 16849648
- PMCID: PMC1895523
- DOI: 10.1182/blood-2005-12-010934
Short-chain fatty acids induce gamma-globin gene expression by displacement of a HDAC3-NCoR repressor complex
Abstract
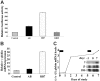
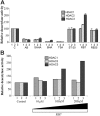
High-level induction of fetal (gamma) globin gene expression for therapy of beta-hemoglobinopathies likely requires local chromatin modification and dissociation of repressor complexes for gamma-globin promoter activation. A novel gamma-globin-inducing short-chain fatty acid derivative (SCFAD), RB7, which was identified through computational modeling, produced a 6-fold induction in a reporter assay that detects only strong inducers of the gamma-globin gene promoter and in cultured human erythroid progenitors. To elucidate the molecular mechanisms used by high-potency SCFADs, chromatin immunoprecipitation (ChIP) assays performed at the human gamma- and beta-globin gene promoters in GM979 cells and in erythroid progenitors demonstrate that RB7 and butyrate induce dissociation of HDAC3 (but not HDAC1 or HDAC2) and its adaptor protein NCoR, specifically from the gamma-globin gene promoter. A coincident and proportional recruitment of RNA polymerase II to the gamma-globin gene promoter was observed with exposure to these gamma-globin inducers. Knockdown of HDAC3 by siRNA induced transcription of the gamma-globin gene promoter, demonstrating that displacement of HDAC3 from the gamma-globin gene promoter by the SCFAD is sufficient to induce gamma-globin gene expression. These studies demonstrate new dynamic alterations in transcriptional regulatory complexes associated with SCFAD-induced activation of the gamma-globin gene and provide a specific molecular target for potential therapeutic intervention.
Figures
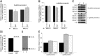
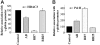
Similar articles
-
Short-chain fatty acid derivatives induce fetal globin expression and erythropoiesis in vivo.Blood. 2002 Dec 15;100(13):4640-8. doi: 10.1182/blood-2002-02-0353. Epub 2002 Aug 15. Blood. 2002. PMID: 12393583 Free PMC article.
-
Hydroxamide derivatives of short-chain fatty acids are potent inducers of human fetal globin gene expression.Exp Hematol. 2003 Mar;31(3):197-203. doi: 10.1016/s0301-472x(02)01030-5. Exp Hematol. 2003. PMID: 12644016
-
Histone deacetylase 9 activates gamma-globin gene expression in primary erythroid cells.J Biol Chem. 2011 Jan 21;286(3):2343-53. doi: 10.1074/jbc.M110.115725. Epub 2010 Nov 13. J Biol Chem. 2011. PMID: 21078662 Free PMC article.
-
Therapeutic fetal-globin inducers reduce transcriptional repression in hemoglobinopathy erythroid progenitors through distinct mechanisms.Blood Cells Mol Dis. 2016 Jan;56(1):62-9. doi: 10.1016/j.bcmd.2015.10.004. Epub 2015 Oct 27. Blood Cells Mol Dis. 2016. PMID: 26603726 Free PMC article.
-
Cellular and molecular effects of a pulse butyrate regimen and new inducers of globin gene expression and hematopoiesis.Ann N Y Acad Sci. 1998 Jun 30;850:87-99. doi: 10.1111/j.1749-6632.1998.tb10466.x. Ann N Y Acad Sci. 1998. PMID: 9668531 Review.
Cited by
-
FK228 Analogues Induce Fetal Hemoglobin in Human Erythroid Progenitors.Anemia. 2012;2012:428137. doi: 10.1155/2012/428137. Epub 2012 May 14. Anemia. 2012. PMID: 22655179 Free PMC article.
-
Histone deacetylase inhibitors and hemoglobin F induction in beta-thalassemia.Int J Biochem Cell Biol. 2008;40(11):2341-7. doi: 10.1016/j.biocel.2008.04.024. Epub 2008 May 29. Int J Biochem Cell Biol. 2008. PMID: 18617435 Free PMC article. Review.
-
Histone Deacetylases Function in the Control of Early Hematopoiesis and Erythropoiesis.Int J Mol Sci. 2022 Aug 29;23(17):9790. doi: 10.3390/ijms23179790. Int J Mol Sci. 2022. PMID: 36077192 Free PMC article. Review.
-
Anti-malarial effect of gum arabic.Malar J. 2011 May 20;10:139. doi: 10.1186/1475-2875-10-139. Malar J. 2011. PMID: 21599958 Free PMC article.
-
Novel Inducers of Fetal Globin Identified through High Throughput Screening (HTS) Are Active In Vivo in Anemic Baboons and Transgenic Mice.PLoS One. 2015 Dec 29;10(12):e0144660. doi: 10.1371/journal.pone.0144660. eCollection 2015. PLoS One. 2015. PMID: 26713848 Free PMC article.
References
-
- Peterson KR. Hemoglobin switching: new insights. Curr Opin Hematol. 2003;10: 123-129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

