Antihypertensive efficacy of Irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetes
- PMID: 16849900
- PMCID: PMC8109400
- DOI: 10.1111/j.1524-6175.2006.05486.x
Antihypertensive efficacy of Irbesartan/HCTZ in men and women with the metabolic syndrome and type 2 diabetes
Abstract
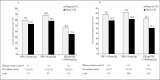
This subgroup analysis of the Irbesartan/Hydrochlorothiazide (HCTZ) Blood Pressure Reductions in Diverse Patient Populations (INCLUSIVE) trial evaluated the efficacy and safety of irbesartan/HCTZ fixed combinations in adults with uncontrolled systolic blood pressure (SBP) (140-159 mm Hg; 130-159 mm Hg for type 2 diabetes mellitus [T2DM]) after >or=4 weeks of antihypertensive monotherapy. Treatment was sequential: placebo (4-5 weeks), HCTZ 12.5 mg (2 weeks), irbesartan/HCTZ 150/12.5 mg (8 weeks), and irbesartan/HCTZ 300/25 mg (8 weeks). In the intent-to-treat analysis, mean change from baseline (end of placebo phase) off all previous therapy to Week 18 (study end) in T2DM patients (n=227) was -18.2+/-14.1 mm Hg for SBP (primary end point; p<0.001) and -8.7+/-8.2 mm Hg for diastolic blood pressure (p<0.001). Mean SBP/diastolic blood pressure changes in patients with the metabolic syndrome (n=345) were -21.0+/-14.3/-10.4+/-8.5 mm Hg (p<0.001). Overall, 56% (95% confidence interval, 49%-62%) of T2DM and 73% (95% confidence interval, 68%-77%) of metabolic syndrome patients achieved SBP goal (<140 mm Hg; <130 mm Hg for T2DM). Goal attainment rates were significantly higher among women with the metabolic syndrome than men. Treatments appeared to be well tolerated. Irbesartan/HCTZ fixed combinations achieved SBP goals in over half of the T2DM patients and nearly three quarters of patients with the metabolic syndrome, with SBP uncontrolled on antihypertensive monotherapy.
Figures
Similar articles
-
Antihypertensive efficacy and tolerability of irbesartan/hydrochlorothiazide in hypertensive patients stratified by body mass index and type 2 diabetes mellitus status: a post hoc subgroup analysis of the Irbesartan/HCTZ Blood Pressure Reductions in Diverse Patient Populations trial.Clin Ther. 2008 Dec;30(12):2354-65. doi: 10.1016/j.clinthera.2008.12.018. Clin Ther. 2008. PMID: 19167594 Clinical Trial.
-
The efficacy and safety of low- and high-dose fixed combinations of irbesartan/hydrochlorothiazide in patients with uncontrolled systolic blood pressure on monotherapy: the INCLUSIVE trial.J Clin Hypertens (Greenwich). 2005 Oct;7(10):578-86. doi: 10.1111/j.1524-6175.2004.04720.x. J Clin Hypertens (Greenwich). 2005. PMID: 16227760 Free PMC article. Clinical Trial.
-
Irbesartan/hydrochlorothiazide for the treatment of isolated systolic hypertension: a subgroup analysis of the INCLUSIVE trial.J Natl Med Assoc. 2009 Apr;101(4):300-7. doi: 10.1016/s0027-9684(15)30876-2. J Natl Med Assoc. 2009. PMID: 19397219 Clinical Trial.
-
Fixed combination of irbesartan and hydrochlorothiazide in the management of hypertension.Vasc Health Risk Manag. 2009;5(1):213-24. doi: 10.2147/vhrm.s3302. Epub 2009 Apr 8. Vasc Health Risk Manag. 2009. PMID: 19436667 Free PMC article. Review.
-
Management of hypertension with fixed dose combinations of candesartan cilexetil and hydrochlorothiazide: patient perspectives and clinical utility.Vasc Health Risk Manag. 2009;5:1043-58. doi: 10.2147/vhrm.s5549. Epub 2009 Dec 29. Vasc Health Risk Manag. 2009. PMID: 20057897 Free PMC article. Review.
Cited by
-
The role of irbesartan in the treatment of patients with hypertension: a comprehensive and practical review.High Blood Press Cardiovasc Prev. 2012 Mar 1;19(1):19-31. doi: 10.2165/11632100-000000000-00000. High Blood Press Cardiovasc Prev. 2012. PMID: 22670584 Review.
-
Strategies for improving cardiovascular health in women with diabetes mellitus: a review of the evidence.Curr Diab Rep. 2015 Nov;15(11):98. doi: 10.1007/s11892-015-0665-7. Curr Diab Rep. 2015. PMID: 26391392 Free PMC article.
-
Efficacy and safety of fixed combinations of irbesartan/hydrochlorothiazide in hypertensive women: the inclusive trial.J Womens Health (Larchmt). 2008 Jul-Aug;17(6):931-8. doi: 10.1089/jwh.2008.0499. J Womens Health (Larchmt). 2008. PMID: 18681815 Free PMC article. Clinical Trial.
-
Effect of the angiotensin receptor blocker irbesartan on metabolic parameters in clinical practice: the DO-IT prospective observational study.Cardiovasc Diabetol. 2007 Nov 27;6:36. doi: 10.1186/1475-2840-6-36. Cardiovasc Diabetol. 2007. PMID: 18042288 Free PMC article. Clinical Trial.
-
Predictors of blood pressure response to angiotensin receptor blocker/diuretic combination therapy: a secondary analysis of the irbesartan/hydrochlorothiazide blood pressure reductions in diverse patient populations (INCLUSIVE) study.J Clin Hypertens (Greenwich). 2008 Jan;10(1):27-33. doi: 10.1111/j.1524-6175.2007.07195.x. J Clin Hypertens (Greenwich). 2008. PMID: 18174768 Free PMC article. Clinical Trial.
References
-
- Sowers JR, Haffner S. Treatment of cardiovascular and renal risk factors in the diabetic hypertensive. Hypertension. 2002;40:781–788. - PubMed
-
- Sowers JR. Treatment of hypertension in patients with diabetes. Arch Intern Med. 2004;164:1850–1857. - PubMed
-
- National Kidney Foundation. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Kidney Disease Outcomes Quality Initiative (K/DOQI). Am J Kidney Dis. 2004;43(5 suppl 1):S1–S290. - PubMed
-
- Ninomiya JK, L'ltalien G, Criqui MH, et al. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. - PubMed
-
- American Diabetes Association . Standards of medical care in diabetes. Diabetes Care. 2005;28(suppl 1):S4–S36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical